Most exhaustion studies have focused on CD8+ T cells. Here, we demonstrated reciprocal growth inhibition of CD4+ T cells and colorectal cancer cells, which induced the expression of PD-1, PD-L1, and PD-L2 in CD4+ T cells. The accelerated exhaustion of CD4+ T cells was evidenced by the reduced secretion of several cytokines, including IL-2, IFN-γ, or TNFα, and elevated secretion of CXCL family chemokines. Progressive expression of PD-L1, CTLA4, and IDO1 exhaustion markers occurred concomitantly with tumor growth in vivo in a mouse model. The pattern of CD4+ T cell exhaustion was analogous to that observed in CD8+ T cells, although with altered dynamics. The PD-L1-high phenotype can be induced by co-culture with tumor cells and is mediated by secreted factors in addition to cell contact. Our findings revealed that IFN-γ receptor knockout T cells exhibited PD-L1 protein expression when cultured with tumor cells, suggesting that PD-L1 expression is not fully dependent on IFN-γ. The TIL population undergoing exhaustion due to persistent antigen stimulation in the presence of cancer cells gradually acquires an immunosuppressive phenotype. The accumulation of inhibitory signals exerted by both cancer cells and T cells, which had converted to a suppressive phenotype, accelerated T cell exhaustion.
Product Citations: 44
Cancer cells accelerate exhaustion of persistently activated mouse CD4+ T cells.
In Oncoimmunology on 1 December 2025 by Stachowiak, M., Becker, W., et al.
-
Cancer Research
-
Immunology and Microbiology
In Cancer Immunology Research on 1 August 2024 by Liao, R., Hsu, J. Y., et al.
The specific BCL-2 small molecule inhibitor venetoclax induces apoptosis in a wide range of malignancies, which has led to rapid clinical expansion in its use alone and in combination with chemotherapy and immune-based therapies against a myriad of cancer types. While lymphocytes, and T cells in particular, rely heavily on BCL-2 for survival and function, the effects of small molecule blockade of the BCL-2 family on surviving immune cells is not fully understood. We aimed to better understand the effect of systemic treatment with venetoclax on regulatory T cells (Treg), which are relatively resistant to cell death induced by specific drugging of BCL-2 compared to other T cells. We found that BCL-2 blockade altered Treg transcriptional profiles and mediated Treg plasticity toward a TH17-like Treg phenotype, resulting in increased IL17A production in lymphoid organs and within the tumor microenvironment. Aligned with previously described augmented antitumor effects observed when combining venetoclax with anti-PD-1 checkpoint inhibition, we also demonstrated that Treg-specific genetic BCL-2 knockout combined with anti-PD-1 induced tumor regression and conferred overlapping genetic changes with venetoclax-treated Tregs. As long-term combination therapies using venetoclax gain more traction in the clinic, an improved understanding of the immune-modulatory effects caused by venetoclax may allow expansion of its use against malignancies and immune-related diseases.
©2024 American Association for Cancer Research.
-
Immunology and Microbiology
The potential role of CMC1 as an immunometabolic checkpoint in T cell immunity.
In Oncoimmunology on 25 April 2024 by Chen, Y., Gao, J., et al.
T cell immunity is critical for human defensive immune response. Exploring the key molecules during the process provides new targets for T cell-based immunotherapies. CMC1 is a mitochondrial electron transport chain (ETC) complex IV chaperon protein. By establishing in-vitro cell culture system and Cmc1 gene knock out mice, we evaluated the role of CMC1 in T cell activation and differentiation. The B16-OVA tumor model was used to test the possibility of targeting CMC1 for improving T cell anti-tumor immunity. We identified CMC1 as a positive regulator in CD8+T cells activation and terminal differentiation. Meanwhile, we found that CMC1 increasingly expressed in exhausted T (Tex) cells. Genetic lost of Cmc1 inhibits the development of CD8+T cell exhaustion in mice. Instead, deletion of Cmc1 in T cells prompts cells to differentiate into metabolically and functionally quiescent cells with increased memory-like features and tolerance to cell death upon repetitive or prolonged T cell receptor (TCR) stimulation. Further, the in-vitro mechanistic study revealed that environmental lactate enhances CMC1 expression by inducing USP7, mediated stabilization and de-ubiquitination of CMC1 protein, in which a mechanism we propose here that the lactate-enriched tumor microenvironment (TME) drives CD8+TILs dysfunction through CMC1 regulatory effects on T cells. Taken together, our study unraveled the novel role of CMC1 as a T cell regulator and its possibility to be utilized for anti-tumor immunotherapy.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Senolytic CAR T cells reverse aging-associated defects in intestinal regeneration and fitness
Preprint on BioRxiv : the Preprint Server for Biology on 22 March 2024 by Eskiocak, O., Chowdhury, S., et al.
SUMMARY Intestinal stem cells (ISCs) drive the rapid regeneration of the gut epithelium to maintain organismal homeostasis. Aging, however, significantly reduces intestinal regenerative capacity. While cellular senescence is a key feature of the aging process, little is known about the in vivo effects of senescent cells on intestinal fitness. Here, we identify the accumulation of senescent cells in the aging gut and, by harnessing senolytic CAR T cells to eliminate them, we uncover their detrimental impact on epithelial integrity and overall intestinal homeostasis in natural aging, injury and colitis. Ablation of intestinal senescent cells with senolytic CAR T cells in vivo or in vitro is sufficient to promote the regenerative potential of aged ISCs. This intervention improves epithelial integrity and mucosal immune function. Overall, these results highlight the ability of senolytic CAR T cells to rejuvenate the intestinal niche and demonstrate the potential of targeted cell therapies to promote tissue regeneration in aging organisms.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Nature Aging on 1 March 2024 by Amor, C., Fernández-Maestre, I., et al.
Senescent cells, which accumulate in organisms over time, contribute to age-related tissue decline. Genetic ablation of senescent cells can ameliorate various age-related pathologies, including metabolic dysfunction and decreased physical fitness. While small-molecule drugs that eliminate senescent cells ('senolytics') partially replicate these phenotypes, they require continuous administration. We have developed a senolytic therapy based on chimeric antigen receptor (CAR) T cells targeting the senescence-associated protein urokinase plasminogen activator receptor (uPAR), and we previously showed these can safely eliminate senescent cells in young animals. We now show that uPAR-positive senescent cells accumulate during aging and that they can be safely targeted with senolytic CAR T cells. Treatment with anti-uPAR CAR T cells improves exercise capacity in physiological aging, and it ameliorates metabolic dysfunction (for example, improving glucose tolerance) in aged mice and in mice on a high-fat diet. Importantly, a single administration of these senolytic CAR T cells is sufficient to achieve long-term therapeutic and preventive effects.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
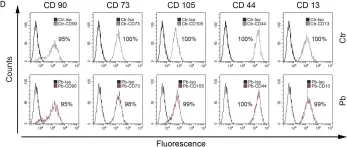
In PLoS One on 27 December 2018 by Griukova, A., Deryabin, P., et al.
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
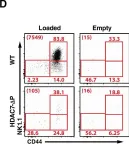
In Elife on 17 April 2018 by Kasler, H. G., Lee, I. S., et al.
Fig.1.D

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 4
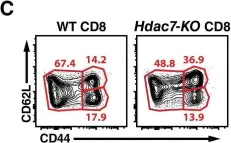
In Elife on 17 April 2018 by Kasler, H. G., Lee, I. S., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 4
In Elife on 17 April 2018 by Kasler, H. G., Lee, I. S., et al.
Fig.2.C

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 4