The poor prognosis of pancreatic ductal adenocarcinoma (PDAC) is largely due to several challenges, such as late diagnosis, early metastasis, limited response to chemotherapy, aggressive tumor biology, and high rates of tumor recurrence. Therefore, the development of a non-invasive and effective method for early detection of PDAC is crucial to improving patient outcomes. Continued research and exploration in this area are essential to enhance early detection methods and ultimately improve the prognosis for individuals with PDAC. In this study, we examined 37 exosomal surface proteins through a multiplex flow cytometry test on peripheral plasma samples from a group of 51 clinical control individuals (including healthy volunteers and non-cancer patients (Cholecystectomy, Hernia, healthy volunteers)), 21 pancreatitis, and 48 patients diagnosed with PDAC. Our research findings revealed that the level of exosomal CD40 expression is significantly lower in patients with PDAC and pancreatitis compared to non-cancer patients (p < 0.0001). Additionally, pancreatitis patients exhibited higher levels of exosomal CD25 expression than PDAC patients (p = 0.0104). PDAC patients with higher exo-CD40 had worse survival than patients with lower exo-CD40 (p = 0.0035). Similarly, PDAC patients with higher exo-CD25 showed worse survival in comparison to patients with lower exo-CD25 (p = 0.04). Statistical analysis revealed that exosomal CD40 achieved an AUC of 0.827 in distinguishing PDAC from clinical controls. Combining exo-CD40 along with exo-CD25 and CA19-9 discriminated PDAC patients from clinical controls with an AUC of 0.92. Exo-CD40 and exo-CD25 proteins found in exosomes isolated from plasma can serve as excellent non-invasive biomarkers for the early diagnosis of PDAC. Further larger scale studies are needed to validate combined exo-CD40 and exo-CD25 as a diagnostic tool for the identification of PDAC patients through non-invasive liquid biopsy.
Product Citations: 88
In International Journal of Molecular Sciences on 11 February 2025 by David, P., Kouhestani, D., et al.
-
Cancer Research
Cholesterol promotes IFNG mRNA expression in CD4+ effector/memory cells by SGK1 activation.
In Life Science Alliance on 1 December 2024 by Hanin, A., Comi, M., et al.
IFNγ-secreting T cells are central for the maintenance of immune surveillance within the central nervous system (CNS). It was previously reported in healthy donors that the T-cell environment in the CNS induces distinct signatures related to cytotoxic capacity, CNS trafficking, tissue adaptation, and lipid homeostasis. These findings suggested that the CNS milieu consisting predominantly of lipids mediated the metabolic conditions leading to IFNγ-secreting brain CD4 T cells. Here, we demonstrate that the supplementation of CD4+CD45RO+CXCR3+ cells with cholesterol modulates their function and increases IFNG expression. The heightened IFNG expression was mediated by the activation of the serum/glucocorticoid-regulated kinase (SGK1). Inhibition of SGK1 by a specific enzymatic inhibitor significantly reduces the expression of IFNG Our results confirm the crucial role of lipids in maintaining T-cell homeostasis and demonstrate a putative role of environmental factors to induce effector responses in CD4+ effector/memory cells. These findings offer potential avenues for further research targeting lipid pathways to modulate inflammatory conditions.
© 2024 Hanin et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Genetics
-
Immunology and Microbiology
Concomitant administration of seasonal influenza and COVID-19 mRNA vaccines.
In Emerging Microbes Infections on 1 December 2024 by Aydillo, T., Balsera-Manzanero, M., et al.
Current clinical guidelines support the concomitant administration of seasonal influenza vaccines and COVID-19 mRNA boosters vaccine. Whether dual vaccination may impact vaccine immunogenicity due to an interference between influenza or SARS-CoV-2 antigens is unknown. We aimed to understand the impact of mRNA COVID-19 vaccines administered concomitantly on the immune response to influenza vaccines. For this, 128 volunteers were vaccinated during the 22-23 influenza season. Three groups of vaccination were assembled: FLU vaccine only (46, 35%) versus volunteers that received the mRNA bivalent COVID-19 vaccines concomitantly to seasonal influenza vaccines, FluCOVID vaccine in the same arm (42, 33%) or different arm (40, 31%), respectively. Sera and whole blood were obtained the day of vaccination, +7, and +28 days after for antibody and T cells response quantification. As expected, side effects were increased in individuals who received the FluCOVID vaccine as compared to FLU vaccine only based on the known reactogenicity of mRNA vaccines. In general, antibody levels were high at 4 weeks post-vaccination and differences were found only for the H3N2 virus when administered in different arms compared to the other groups at day 28 post-vaccination. Additionally, our data showed that subjects that received the FluCOVID vaccine in different arm tended to have better antibody induction than those receiving FLU vaccines for H3N2 virus in the absence of pre-existing immunity. Furthermore, no notable differences in the influenza-specific cellular immune response were found for any of the vaccination groups. Our data supports the concomitant administration of seasonal influenza and mRNA COVID-19 vaccines.
-
COVID-19
-
Genetics
-
Immunology and Microbiology
In Heliyon on 15 October 2024 by Zhu, A., Zhou, L., et al.
The vastly spreading COVID-19 pneumonia is caused by SARS-CoV-2. Lymphopenia and cytokine levels are tightly associated with disease severity. However, virus-induced immune dysregulation at cellular and molecular levels remains largely undefined. Here, the leukocytes in the pleural effusion, sputum, and peripheral blood biopsies from severe and mild patients were analyzed at single-cell resolution. Drastic T cell hyperactivation accompanying elevated T cell exhaustion was observed, predominantly in pleural effusion. The mechanistic investigation identified a group of CD14+ monocytes and macrophages highly expressing CD163 and MRC1 in the biopsies from severe patients, suggesting M2 macrophage polarization. These M2-like cells exhibited up-regulated IL10, CCL18, APOE, CSF1 (M-CSF), and CCL2 signaling pathways. Further, cell type specific dysregulation of transposable elements was observed in Severe COVID-19 patients. Together, our results suggest that severe SARS-CoV-2 infection causes immune dysregulation by inducing M2 polarization and subsequent T cell exhaustion. This study improves our understanding of COVID-19 pathogenesis.
© 2024 The Authors. Published by Elsevier Ltd.
-
COVID-19
-
Immunology and Microbiology
Crohn's disease treatment and memory T-cell subset changes: insights from a case series.
In Translational Gastroenterology and Hepatology on 8 May 2024 by Chen, Z. H., Tang, Y. Y., et al.
Crohn's disease (CD) is a chronic inflammatory bowel disease with significant morbidity, affecting millions worldwide. The intricacies of immune responses in CD, especially post-treatment, remain a vital area of exploration. While memory T (Tm)-cell subsets play a pivotal role in adaptive immunity, their specific function in patients with CD after treatment is not well-understood. This study aims to investigate the effect and function of Tm-cell subsets in these patients, addressing a crucial knowledge gap in the context of CD therapeutics.
A total of eight patients diagnosed with CD were selected based on predefined inclusion criteria. All patients were treated with either anti-inflammatory agents, immunosuppressive drugs, or a combination of both. For comparison, healthy donors were enrolled based on exclusion of autoimmune or inflammatory diseases. Peripheral blood mononuclear cells (PBMCs) and lymphocytes were isolated from blood and lymph node tissue respectively. The phenotype and cytokine production of T lymphocytes from both CD patients and healthy donors were analyzed using flow cytometry. Statistical comparisons of the outcomes between CD patients and healthy donors were made using Mann-Whitney test (two-tailed) and Student t-test.
Post-treatment CD patients exhibited an altered T cell distribution with a notable increase in CD8+ T cells in PBMCs (P=0.0005), and altered frequencies of CD4+ and CD8+ T cells in mesenteric lymph nodes (MLNs). Tm cells showed decreased interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) production, with significant alterations in the frequency of IFN-γ-producing CD8+ stem cell-like Tm (Tscm) cells in lesions of the MLNs from patients with CD (CD-M-Lys) compared to healthy MLNs from patients with CD (N-M-Lys) (P=0.0152). Differences in tissue-resident Tm (Trm)-cell subset frequencies were observed between the MLNs and small intestinal mucosa in CD patients.
The treatments with anti-inflammatory agents and/or immunosuppressive drugs have a significant effect on the frequency and function of Tm-cell subsets. Clinically, these findings suggest a potential therapeutic avenue in modulating Tm-cell responses, which might be particularly beneficial for conditions where immune response modulation is crucial. Further clinical studies are warranted to explore the full therapeutic implications of these findings.
2024 Translational Gastroenterology and Hepatology. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
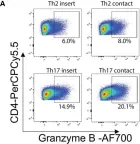
In Front Immunol on 29 April 2022 by Jamann, H., Cui, Q. L., et al.
Fig.4.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
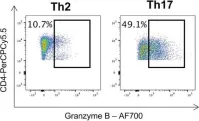
In Front Immunol on 29 April 2022 by Jamann, H., Cui, Q. L., et al.
Fig.3.E

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
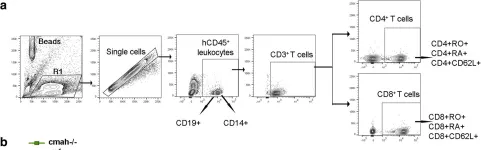
In BMC Immunol on 7 January 2019 by Dagur, R. S., Branch-Woods, A., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
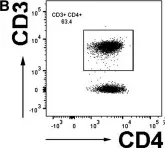
In Immunol Res on 1 December 2015 by Monahan, R., Stein, A., et al.
Fig.3.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 6
In Immunol Res on 1 December 2015 by Monahan, R., Stein, A., et al.
Fig.1.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 6
In Immunol Res on 1 December 2015 by Monahan, R., Stein, A., et al.
Fig.1.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 6