In 2022, a global mpox outbreak occurred, and remains a concern today. The T cell memory response to MPXV (monkeypox virus) infection has not been fully investigated. In this study, we evaluate this response in convalescent and MVA-BN (Modified Vaccinia Ankara - Bavarian Nordic) vaccinated individuals using VACV-infected cells. Strong CD8+ and CD4+ T cell responses are observed, and T cell responses are biased towards viral early expressed proteins. We identify seven immunodominant HLA-A*02:01 restricted MPXV-specific epitopes and focus our detailed phenotypic and scRNAseq analysis on the immunodominant HLA-A*02:01-G5R18-26-specific CD8+ T cell response. While tetramer+CD8+ T cells share similar differentiation and activation phenotypes, T cells from convalescent individuals show greater cytotoxicity, migratory potential to site of infection and TCR clonal expansion. Our data suggest that effective functional profiles of MPXV-specific memory T cells induced by Mpox infection may have an implication on the long-term protective responses to future infection.
© 2025. The Author(s).
Product Citations: 65
In Nature Communications on 10 May 2025 by Chen, J. L., Wang, B., et al.
-
Homo sapiens (Human)
-
Immunology and Microbiology
Regulated GATA1 expression as a universal gene therapy for Diamond-Blackfan anemia.
In Cell Stem Cell on 2 January 2025 by Voit, R. A., Liao, X., et al.
Gene therapy using hematopoietic stem and progenitor cells is altering the therapeutic landscape for patients with hematologic, immunologic, and metabolic disorders but has not yet been successfully developed for individuals with the bone marrow failure syndrome Diamond-Blackfan anemia (DBA). More than 30 mutations cause DBA through impaired ribosome function and lead to inefficient translation of the erythroid master regulator GATA1, providing a potential avenue for therapeutic intervention applicable to all patients with DBA, irrespective of the underlying genotype. Here, we report the development of a clinical-grade lentiviral gene therapy that achieves erythroid lineage-restricted expression of GATA1. We show that this vector is capable of augmenting erythropoiesis in DBA models and diverse patient samples without impacting hematopoietic stem cell function or demonstrating any signs of premalignant clonal expansion. These preclinical safety and efficacy data provide strong support for the first-in-human universal gene therapy trial for DBA through regulated GATA1 expression.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Stem Cells and Developmental Biology
In Nature Medicine on 1 December 2024 by Sáez-Cirión, A., Mamez, A. C., et al.
HIV cure has been reported for five individuals who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) with cells from CCR5Δ32 homozygous donors. By contrast, viral rebound has occurred in other people living with HIV who interrupted antiretroviral treatment after undergoing allo-HSCT, with cells mostly from wild-type CCR5 donors. Here we report the case of a male individual who has achieved durable HIV remission following allo-HSCT with cells from an unrelated HLA-matched (9 of 10 matching for HLA-A, HLA-B, HLA-C, HLA-DRB1 and HLA-DQB1 alleles) wild-type CCR5 donor to treat an extramedullary myeloid tumor. To date, plasma viral load has remained undetectable for 32 months after the interruption of antiretroviral treatment. Treatment with ruxolitinib has been maintained during this period to treat chronic graft-versus-host disease. Low levels of proviral DNA were detected sporadically after allo-HSCT, including defective but not intact HIV DNA. No virus could be amplified in cultures of CD4+ T cells obtained after antiretroviral treatment interruption, while CD4+ T cells remained susceptible to HIV-1 infection in vitro. Declines in HIV antibodies and undetectable HIV-specific T cell responses further corroborate the absence of viral rebound after antiretroviral treatment interruption. These results suggest that HIV remission could be achieved in the context of allo-HSCT with wild-type CCR5.
© 2024. The Author(s).
-
Stem Cells and Developmental Biology
In IScience on 20 September 2024 by Fert, A., Richard, J., et al.
The mechanistic target of rapamycin (mTOR) positively regulates multiple steps of the HIV-1 replication cycle. We previously reported that a 12-week supplementation of antiretroviral therapy (ART) with metformin, an indirect mTOR inhibitor used in type-2 diabetes treatment, reduced mTOR activation and HIV transcription in colon-infiltrating CD4+ T cells, together with systemic inflammation in nondiabetic people with HIV-1 (PWH). Herein, we investigated the antiviral mechanisms of metformin. In a viral outgrowth assay performed with CD4+ T cells from ART-treated PWH, and upon infection in vitro with replication-competent and VSV-G-pseudotyped HIV-1, metformin decreased virion release, but increased the frequency of productively infected CD4lowHIV-p24+ T cells. These observations coincided with increased BST2/tetherin (HIV release inhibitor) and Bcl-2 (pro-survival factor) expression, and improved recognition of productively infected T cells by HIV-1 envelope antibodies. Thus, metformin exerts pleiotropic effects on post-integration steps of the HIV-1 replication cycle and may be used to accelerate viral reservoir decay in ART-treated PWH.
© 2024 The Authors.
-
Immunology and Microbiology
FLT3L governs the development of partially overlapping hematopoietic lineages in humans and mice.
In Cell on 23 May 2024 by Momenilandi, M., Levy, R., et al.
FMS-related tyrosine kinase 3 ligand (FLT3L), encoded by FLT3LG, is a hematopoietic factor essential for the development of natural killer (NK) cells, B cells, and dendritic cells (DCs) in mice. We describe three humans homozygous for a loss-of-function FLT3LG variant with a history of various recurrent infections, including severe cutaneous warts. The patients' bone marrow (BM) was hypoplastic, with low levels of hematopoietic progenitors, particularly myeloid and B cell precursors. Counts of B cells, monocytes, and DCs were low in the patients' blood, whereas the other blood subsets, including NK cells, were affected only moderately, if at all. The patients had normal counts of Langerhans cells (LCs) and dermal macrophages in the skin but lacked dermal DCs. Thus, FLT3L is required for B cell and DC development in mice and humans. However, unlike its murine counterpart, human FLT3L is required for the development of monocytes but not NK cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
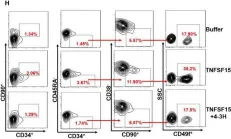
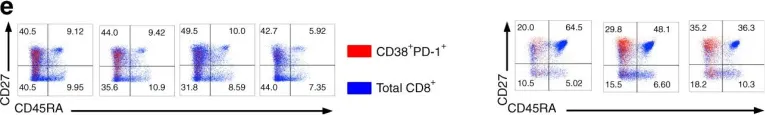
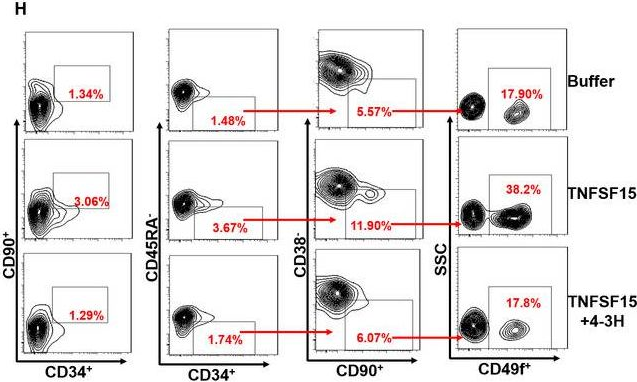
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.1.H
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
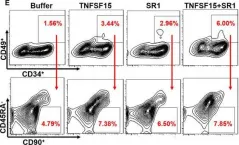
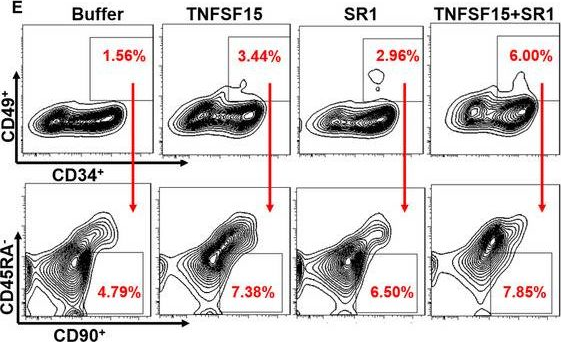
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.3.E
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
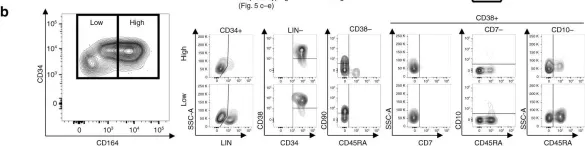
In Nat Commun on 3 June 2019 by Pellin, D., Loperfido, M., et al.
Fig.5.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 5
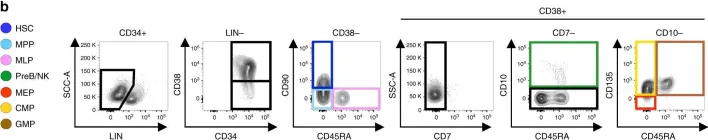
In Nat Commun on 3 June 2019 by Pellin, D., Loperfido, M., et al.
Fig.1.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 5
In Nat Commun on 26 February 2018 by Wang, Z., Zhu, L., et al.
Fig.2.E

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 5