BH3 mimetic drugs that selectively target the pro-survival BCL2 proteins are highly promising for cancer treatment, most notably for treating blood cancers. Venetoclax, which inhibits BCL2, is now approved for treating chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). Preferably, robust and validated assays would identify patients most likely to benefit from therapy with venetoclax itself or with inhibitors of other pro-survival proteins. A sophisticated method that has been developed is the BH3 profiling assay. In this assay, permeabilized, instead of intact, cells are treated for a few hours with inhibitors of the pro-survival BCL2 proteins, and the resultant mitochondrial depolarization measured. Sensitivity to a specific inhibitor (e.g., venetoclax or other BH3 mimetics) is then used to infer the reliance of a tumor (e.g., CLL) on one or more pro-survival BCL2 proteins. However, we found that this methodology cannot reliably identify such dependencies. In part, this is because almost all cells express multiple pro-survival BCL2 proteins that restrain BAX and BAK which must be inhibited before mitochondrial depolarization and apoptosis can proceed. Using genetic and pharmacological tools across multiple cell line models of blood cancer, we demonstrated that selective BCL2 inhibitors have important flow-on effects that includes the redistribution of BH3-only proteins to ancillary pro-survival proteins not directly engaged by the inhibitor. These secondary effects, critical to the biological action of selective inhibitors, were not accurately recapitulated in permeabilized cells, probably due to the limited time frame possible in such assays or the altered biophysical conditions when cells are permeabilized. While we could consistently define the sensitivity of a tumor cell to a particular BH3 mimetic drugs using intact cells, this was not reliable with permeabilized cells. These studies emphasize the need to carefully evaluate assays on permeabilized cells undertaken with inhibitors of the pro-survival BCL2 proteins.
© 2025. Crown.
Product Citations: 48
In Cell Death and Differentiation on 9 April 2025 by Gong, J., Djajawi, T. M., et al.
-
Cancer Research
-
Cardiovascular biology
-
Cell Biology
In Frontiers in Immunology on 25 March 2025 by Imamichi, T., Yang, J., et al.
Interleukin (IL)-27 is an anti-viral cytokine. IL-27-treated monocyte-derived macrophages (27-Mac) suppressed HIV replication. Macrophages are generally divided into two subtypes, M1 and M2 macrophages. M2 macrophages can be polarized into M2a, M2b, M2c, and M2d by various stimuli. IL-6 and adenosine induce M2d macrophages. Since IL-27 is a member of the IL-6 family of cytokines, 27-Mac was considered M2d macrophages. In the current study, we compared biological function and gene expression profiles between 27-Mac and M2d subtypes.
Monocytes derived from health donors were differentiated to M2 using macrophage colony-stimulating factor. Then, the resulting M2 was polarized into different subtypes using IL-27, IL-6, or BAY60-658 (an adenosine analog). HIV replication was monitored using a p24 antigen capture assay, and the production of reactive oxygen species (ROS) was determined using a Hydrogen Peroxide Assay. Phagocytosis assay was run using GFP-labeled opsonized E. coli. Cytokine production was detected by the IsoPlexis system, and the gene expression profiles were analyzed using single-cell RNA sequencing (scRNA-seq).
27-Mac and BAY60-658-polarized M2d (BAY-M2d) resisted HIV infection, but IL-6-polarized M2d (6-M2d) lacked the anti-viral effect. Although phagocytosis activity was comparable among the three macrophages, only 27-Mac, but neither 6-M2d nor BAY-M2d, enhanced the generation of ROS. The cytokine-producing profile of 27-Mac did not resemble that of the two subtypes. The scRNA-seq revealed that 27-Mac exhibited a different clustering pattern compared to other M2ds, and each 27-Mac expressed a distinct combination of anti-viral genes. Furthermore, 27-Mac did not express the biomarkers of M2a, M2b, and M2c. However, it significantly expressed CD38 (p<0.01) and secreted CXCL9 (p<0.001), which are biomarkers of M1.
These data suggest that 27-Mac may be classified as either an M1-like subtype or a novel subset of M2, which resists HIV infection mediated by a different mechanism in individual cells using different anti-viral gene products. Our results provide a new insight into the function of IL-27 and macrophages.
Copyright © 2025 Imamichi, Yang, Chen, Goswami, Marquez, Kariyawasam, Sharma, Wiscovitch-Russo, Li, Aioi, Adelsberger, Chang, Higgins and Sui.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Nature Communications on 15 November 2024 by Dieudonné, Y., Lorenzetti, R., et al.
Primary antiphospholipid syndrome (PAPS) is a life-threatening clotting disorder mediated by pathogenic autoantibodies. Here we dissect the origin of self-reactive B cells in human PAPS using peripheral blood and bone marrow of patients with triple-positive PAPS via combined single-cell RNA sequencing, B cell receptors (BCR) repertoire profiling, CITEseq analysis and single cell immortalization. We find that antiphospholipid (aPL)-specific B cells are present in the naive compartment, polyreactive, and derived from the natural repertoire. Furthermore, B cells with aPL specificities are not eliminated in patients with PAPS, persist until the memory and long-lived plasma cell stages, likely after defective germinal center selection, while becoming less polyreactive. Lastly, compared with the non-PAPS cells, PAPS B cells exhibit distinct IFN and APRIL signature as well as dysregulated mTORC1 and MYC pathways. Our findings may thus elucidate the survival mechanisms of these autoreactive B cells and suggest potential therapeutic targets for the treatment of PAPS.
© 2024. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Viruses on 14 October 2024 by Yero, A., Shi, T., et al.
HIV infection significantly affects the frequencies and functions of immunoregulatory CD3+CD4-CD8- double-negative (DN) T-cells, while the effect of early antiretroviral therapy (ART) initiation on these cells remains understudied. DN T-cell subsets were analyzed prospectively in 10 HIV+ individuals during acute infection and following early ART initiation compared to 20 HIV-uninfected controls. In this study, 21 Rhesus macaques (RMs) were SIV-infected, of which 13 were assessed during acute infection and 8 following ART initiation four days post-infection. DN T-cells and FoxP3+ DN Treg frequencies increased during acute HIV infection, which was not restored by ART. The expression of activation (HLA-DR/CD38), immune checkpoints (PD-1/CTLA-4), and senescence (CD28-CD57+) markers by DN T-cells and DN Tregs increased during acute infection and was not normalized by ART. In SIV-infected RMs, DN T-cells remained unchanged despite infection or ART, whereas DN Treg frequencies increased during acute SIV infection and were not restored by ART. Finally, frequencies of CD39+ DN Tregs increased during acute HIV and SIV infections and remained elevated despite ART. Altogether, acute HIV/SIV infections significantly changed DN T-cell and DN Treg frequencies and altered their immune phenotype, while these changes were not fully normalized by early ART, suggesting persistent HIV/SIV-induced immune dysregulation despite early ART initiation.
-
Immunology and Microbiology
CD28 Costimulation Augments CAR Signaling in NK Cells via the LCK/CD3ζ/ZAP70 Signaling Axis.
In Cancer Discovery on 4 October 2024 by Acharya, S., Basar, R., et al.
Multiple factors in the design of a chimeric antigen receptor (CAR) influence CAR T-cell activity, with costimulatory signals being a key component. Yet, the impact of costimulatory domains on the downstream signaling and subsequent functionality of CAR-engineered natural killer (NK) cells remains largely unexplored. Here, we evaluated the impact of various costimulatory domains on CAR-NK cell activity, using a CD70-targeting CAR. We found that CD28, a costimulatory molecule not inherently present in mature NK cells, significantly enhanced the antitumor efficacy and long-term cytotoxicity of CAR-NK cells both in vitro and in multiple xenograft models of hematologic and solid tumors. Mechanistically, we showed that CD28 linked to CD3ζ creates a platform that recruits critical kinases, such as lymphocyte-specific protein tyrosine kinase (LCK) and zeta-chain-associated protein kinase 70 (ZAP70), initiating a signaling cascade that enhances CAR-NK cell function. Our study provides insights into how CD28 costimulation enhances CAR-NK cell function and supports its incorporation in NK-based CARs for cancer immunotherapy. Significance: We demonstrated that incorporation of the T-cell-centric costimulatory molecule CD28, which is normally absent in mature natural killer (NK) cells, into the chimeric antigen receptor (CAR) construct recruits key kinases including lymphocyte-specific protein tyrosine kinase and zeta-chain-associated protein kinase 70 and results in enhanced CAR-NK cell persistence and sustained antitumor cytotoxicity.
©2024 American Association for Cancer Research.
-
Cancer Research
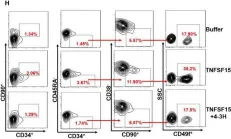
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.1.H
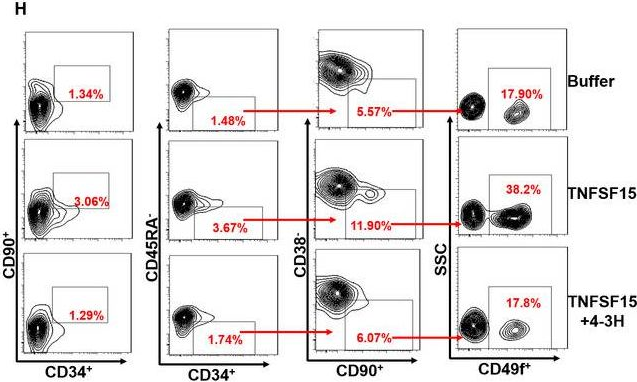
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 1