Activated immune cells infiltrate the vasculature during the pathophysiology of hypertension by establishing a vascular-immune interface that contributes to blood pressure dysregulation and organ failure. Many observations indicate a key role of CD8+ T cells in hypertension but mechanisms regulating their activation and interplay with the cardiovascular system are still unknown. In murine model, here we show that a specific member of the phosphoinositide-3-kinases (PI3K) family of lipid kinases, PI3Kγ, is a key intracellular signaling of CD8+ T cells activation and RANTES/CCL5 secretion in hypertension: CCL5-CCR5 signaling is crucial for the establishment of the vascular-immune interface in peripheral organs, lastly contributing to CD8+ tissue infiltration, organ dysfunction and blood pressure elevation. Our studies identify PI3Kγ as a booster of effector CD8+ T cell function, even in the absence of external stimuli. Lastly, an enhanced PI3Kγ signaling mediates the bystander activation of CD8+ T cells and proves effective in transferring the hypertensive phenotype between mice.
© 2025. The Author(s).
Product Citations: 31
In Nature Communications on 1 July 2025 by Perrotta, M., Perrotta, S., et al.
-
Cardiovascular biology
-
Immunology and Microbiology
In IScience on 15 November 2024 by Luan, Y., Min, Q., et al.
MRL/lpr mice develop systemic lupus erythematosus-like autoimmunity due to defective FAS-mediated apoptosis. We generated Fas lpr mice deficient in EAF2, a transcription elongation-associated factor known to promote apoptosis in germinal center (GC) B cells and crucial for preventing autoimmunity. Contrary to expectations, EAF2 deficiency significantly reduced lymphadenopathy and splenomegaly, extended lifespan, and alleviated nephritis by decreasing renal immune complex deposition. Additionally, EAF2 deficiency markedly reduced accumulation of activated B cells, GC B cells, plasma cells, and the abnormal B220+CD3+ T cells in Fas lpr mice. Further analysis revealed that Eaf2 -/- Fas lpr B cells showed hyperactivation upon various stimulations, followed by increased death. RNA sequencing of the B220+CD3+ cells revealed a downregulation in survival-promoting genes such as Bcl-2 and Akt and an upregulation of proapoptotic genes. We conclude that the combined deficiency in FAS- and EAF2-mediated apoptotic pathways leads to B cell hyperactivation and subsequent death, thereby ameliorating systemic autoimmunity in this model.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Non-homogenous intratumor ionizing radiation doses synergize with PD1 and CXCR2 blockade.
In Nature Communications on 14 October 2024 by Bergeron, P., Dos Santos, M., et al.
The efficacy and side effects of radiotherapy (RT) depend on parameters like dose and the volume of irradiated tissue. RT induces modulations of the tumor immune microenvironment (TIME) that are dependent on the dose. Low dose RT (LDRT, i.e., single doses of 0.5-2 Gy) has been shown to promote immune infiltration into the tumor. Here we hypothesize that partial tumor irradiation combining the immunostimulatory/non-lethal properties of LDRT with cell killing/shrinkage properties of high dose RT (HDRT) within the same tumor mass could enhance anti-tumor responses when combined with immunomodulators. In models of colorectal and breast cancer in immunocompetent female mice, partial irradiation (PI) with millimetric precision to deliver LDRT (2 Gy) and HDRT (16 Gy) within the same tumor induces substantial tumor control when combined with anti-PD1. Using flow cytometry, cytokine profiling and single-cell RNA sequencing, we identify a crosstalk between the TIME of the differentially irradiated tumor volumes. PI reshapes tumor-infiltrating CD8+ T cells into more cytotoxic and interferon-activated phenotypes but also increases the infiltration of pro-tumor neutrophils driven by CXCR2. The combination of the CXCR2 antagonist SB225002 with PD1 blockade and PI improves tumor control and mouse survival. Our results suggest a strategy to reduce RT toxicity and improve the therapeutic index of RT and immune checkpoint combinations.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
In Communications Biology on 17 September 2024 by Wei, P., Romanò, C., et al.
Diseases caused by S. pneumoniae are the leading cause of child mortality. As antibiotic resistance of S. pneumoniae is rising, vaccination remains the most recommended solution. However, the existing pneumococcal polysaccharides vaccine (Pneumovax® 23) proved only to induce T-independent immunity, and strict cold chain dependence of the protein conjugate vaccine impedes its promotion in developing countries, where infections are most problematic. Affordable and efficient vaccines against pneumococcus are therefore in high demand. Here, we present an intranasal vaccine Lipo+CPS12F&αGC, containing the capsular polysaccharides of S. pneumoniae 12F and the iNKT agonist α-galactosylceramide in cationic liposomes. In BALB/cJRj mice, the vaccine effectively activates iNKT cells and promotes B cells maturation, stimulates affinity-matured IgA and IgG production in both the respiratory tract and systemic blood, and displays sufficient protection both in vivo and in vitro. The designed vaccine is a promising, cost-effective solution against pneumococcus, which can be expanded to cover more serotypes and pathogens.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In EMBO Reports on 1 August 2024 by Monticone, G., Huang, Z., et al.
The COVID-19 pandemic reminded us of the urgent need for new antivirals to control emerging infectious diseases and potential future pandemics. Immunotherapy has revolutionized oncology and could complement the use of antivirals, but its application to infectious diseases remains largely unexplored. Nucleoside analogs are a class of agents widely used as antiviral and anti-neoplastic drugs. Their antiviral activity is generally based on interference with viral nucleic acid replication or transcription. Based on our previous work and computer modeling, we hypothesize that antiviral adenosine analogs, like remdesivir, have previously unrecognized immunomodulatory properties which contribute to their therapeutic activity. In the case of remdesivir, we here show that these properties are due to its metabolite, GS-441524, acting as an Adenosine A2A Receptor antagonist. Our findings support a new rationale for the design of next-generation antiviral agents with dual - immunomodulatory and intrinsic - antiviral properties. These compounds could represent game-changing therapies to control emerging viral diseases and future pandemics.
© 2024. The Author(s).
-
COVID-19
-
Genetics
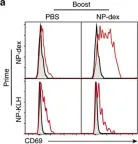
In Nat Commun on 7 December 2016 by Blanc, P., Moro-Sibilot, L., et al.
Fig.6.A

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1