Preclinical studies provided convincing evidence that umbilical cord derived mesenchymal stem cells (UC-MSC) prevent lung injury and promote lung regeneration. We hypothesized that cyclic mechanical stretch (CMS) and hyperoxia (HOX) during mechanical ventilation account for their limited therapeutic efficacy within the clinics. UC-MSC cultures were subjected to CMS and HOX and evaluated for proliferation, cell viability and further functional properties. Reversibility of the phenotype changes was evaluated after recovery in room air following these exposures. CMS and HOX compromised cell viability and proliferation, altered phenotypic characteristics, particularly PDGFRα expression, and induced cellular senescence in UC-MSC. Effects were most pronounced for CMS plus HOX. The alterations of UC-MSC were mediated by p21 accumulation. As inhibition of p21 aggravated cell death of UC-MSC, the results indicated a cell defense mechanism to ensure survival. This assumption was underpinned by the principal reversibility of the phenotype alterations and regrowth after removal of CMS and HOX. But prolonged strongest exposures resulted in definite phenotype changes. CMS and HOX have comparable effects on UC-MSC as described for lung resident MSC. Our results explain their timely limited presence in the diseased lung after therapeutic application. Future research should therefore focus on their repetitive application.
© 2025. The Author(s).
Product Citations: 114
In Scientific Reports on 7 October 2025 by Goetz, M. J., Behnke, J., et al.
-
FC/FACS
In STAR Protocols on 19 September 2025 by Pons, J., Gardet, M., et al.
Dendritic cells (DCs) encompass several subsets that are essential for shaping immune responses. Here, we present a protocol for ex vivo functional analysis and purification of DC subsets from blood and secondary lymphoid organs using flow cytometry. We describe the steps for isolating mononuclear cells from blood, lymph nodes, and spleen. We then detail the procedures for phenotypic characterization of DC subsets, intracellular staining to assess their cytokine production, and fluorescence-activated cell sorting (FACS) to isolate individual DC populations. For complete details on the use and execution of this protocol, please refer to Gardet et al.1.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cardiovascular biology
-
Immunology and Microbiology
In Hematological Oncology on 1 September 2025 by Ovejero, S., Devin, J., et al.
Diffuse large B-cell lymphoma (DLBCL) is the most common hematological malignancy. More than half of DLBCL patients achieve long-term remission after treatment, but a third relapse after conventional Rituximab (R)-based chemotherapy regimens, such as CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone). Cancer cells are exposed to chronic replication stress, which impedes the duplication of their genome. Functional DNA repair pathways are therefore important for the survival of cancer cells. This dependence can be exploited therapeutically to hamper repair of the intrinsic DNA damage occurring during replication or to exacerbate DNA damage induced by chemotherapy. Using CRISPR-Cas9 screening, we identified CHEK1, WEE1, ATR and RAD51 DNA repair factors as essential genes in DLBCL cells. According to these results, we investigated the effect of small molecules targeting DNA replication stress and DNA repair mechanisms, alone or in combination with the R-CHOP genotoxic agents, cyclophosphamide and doxorubicin. Applying a standard threshold of 2 SDs below the IC50 of the genotoxic agent alone, a total of 3 synthetic lethal combinations have been identified including cyclophosphamide with CHK1/2 inhibitor, cyclophosphamide and ATR inhibitor and doxorubicin with DNAPK inhibitor. Co-treatment with these molecules led to cell death, DNA damage induction and cell cycle arrest in DLBCL cells more efficiently than genotoxic agents alone. These data have been validated using primary DLBCL cells from patients. Our results open new perspectives for therapeutic approaches exploiting the synthetic lethality of genotoxic agents with DNA repair inhibitors to improve the therapeutic outcome of patients with DLBCL.
© 2025 The Author(s). Hematological Oncology published by John Wiley & Sons Ltd.
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In Nature Immunology on 1 August 2025 by Venzin, V., Beccaria, C. G., et al.
Chronic hepatitis B virus (HBV) infection is marked by dysfunctional HBV-specific CD8+ T cells, and restoring their effector activity is a major therapeutic goal. Here, we generated HBV-specific CD4+ T cell receptor transgenic mice to show that CD4+ effector T cells can prevent and reverse the CD8⁺ T cell dysfunction induced by hepatocellular priming. This rescue enhances antiviral CD8+ T cell function and suppresses viral replication. CD4+ T cell help occurs directly within the liver, independent of secondary lymphoid organs, and requires local antigen recognition. Kupffer cells, rather than dendritic cells, are the critical antigen-presenting platform. CD4+ T cells license Kupffer cells via CD40-CD40L interactions, triggering interleukin (IL)-12 and IL-27 production. IL-12 expands the CD4+ T cell pool, while IL-27 is essential for CD8+ T cell rescue. Exogenous IL-27 similarly restores HBV-specific CD8+ T cell function in mice and in T cells isolated from chronically infected patients. These findings identify IL-27 as a tractable immunotherapeutic target in chronic HBV infection.
© 2025. The Author(s).
-
FC/FACS
-
Immunology and Microbiology
In Cell Reports Medicine on 15 July 2025 by Yang, Y., Guo, Y., et al.
Clostridium butyricum MIYAIRI 588 (CBM588) has been used to treat gastrointestinal disorders and recently found to enhance efficacy of checkpoint inhibitor in solid tumors. However, limited studies spotlight the role of CBM588 in perioperative management of colorectal cancer (CRC) radical surgery. Here, we report a phase 4, open-label, randomized controlled trial (ChiCTR2000033883) to assess the safety and short-term efficacy of CBM588 as a perioperative, adjunctive therapy. The primary efficacy indicator is postoperative intestinal function assessed by time to first flatus, recovery time of defecation, and first intake of delicate fluid and semi-fluid. Secondary outcomes are incidence of postoperative complications and immune status. We find that perioperative administration of CBM588 significantly benefits recovery of intestinal function, lowers risk of infectious complications, and enhances systemic immunity by increasing circulating T cells. CBM588 serves as a promising probiotic with favorable tolerability in CRC patients undergoing radical surgery.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Cancer Research
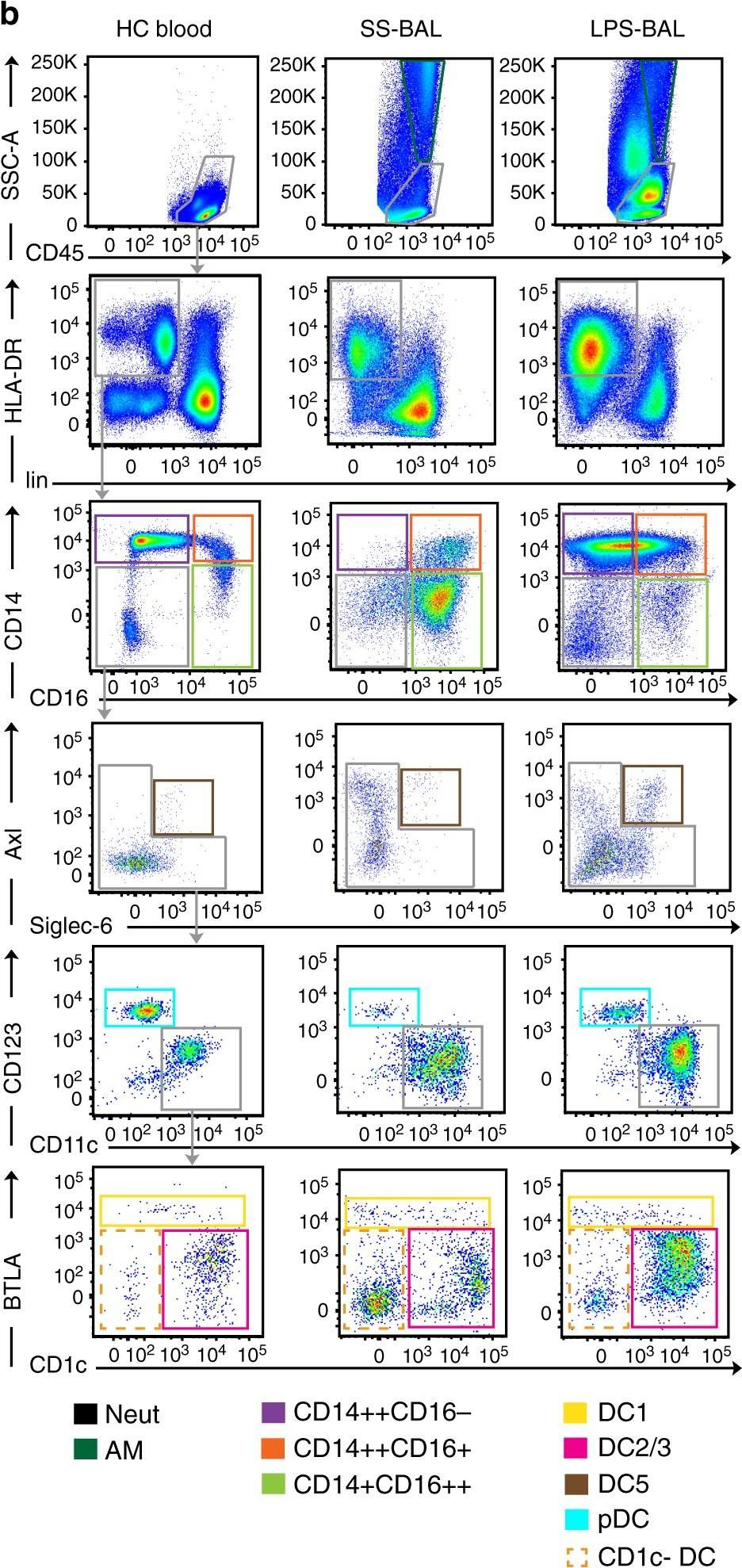
In Nat Commun on 30 April 2019 by Jardine, L., Wiscombe, S., et al.
Fig.1.B
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 4
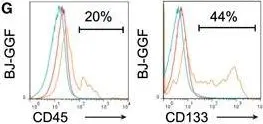
In Cell Rep on 4 December 2018 by Gomes, A. M., Kurochkin, I., et al.
Fig.1.G
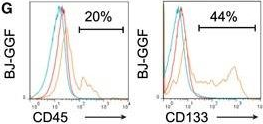
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 4
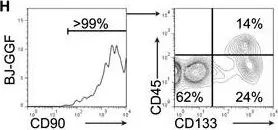
In Cell Rep on 4 December 2018 by Gomes, A. M., Kurochkin, I., et al.
Fig.1.H
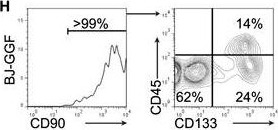
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 4
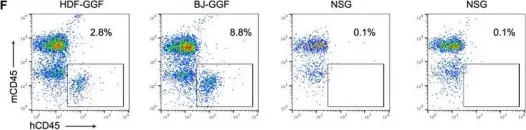
In Cell Rep on 4 December 2018 by Gomes, A. M., Kurochkin, I., et al.
Fig.3.F
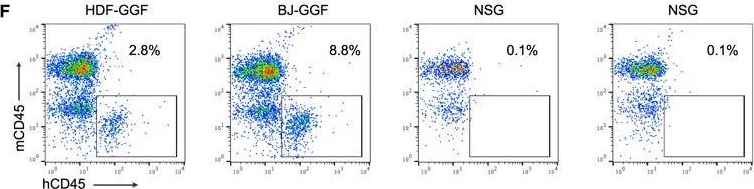
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 4