Breast cancer (BC) is commonly labeled a "cold tumor" due to its dense population of immunosuppressive cells, particularly M2-like macrophages, which contribute to its resistance to therapy. Thus, there is a pressing need to shift the macrophage polarization towards M1 and revitalize the tumor immune microenvironment (TIME) to improve BC prognosis. In this study, we leveraged published RNA-sequencing data and performed multiplex immunohistochemistry on clinical specimens to identify NR4A3 as a promising biomarker for favorable outcomes in BC. High NR4A3 expression correlates with an inflamed TIME, characterized by heightened T-cell infiltration and activation. NR4A3 was preferentially expressed in macrophages and fostered M1-like macrophage polarization through direct binding to p65, thereby enhancing NF-κB transcriptional activity. Overexpression of Nr4a3 in tumor-infiltrating macrophages significantly inhibited the growth of E0771 tumors in a syngeneic mouse model, accompanied by increased T-cell infiltration and elevated production of functional cytokines. Conversely, suppression of Nr4a3 in macrophages compromised T-cell recruitment and diminished their anti-tumor capabilities. Consistent with these findings, co-culture experiments involving human T cells and NR4A3-overexpressing THP1 cells further demonstrated enhanced T-cell functionality. Collectively, our findings uncover a novel role for NR4A3 in macrophage polarization and TIME remodeling, offering a potential biomarker for favorable BC prognosis and a therapeutic target to enhance immunotherapy efficacy.
© 2025. The Author(s).
Product Citations: 61
In NPJ Breast Cancer on 7 July 2025 by Qian, Y. Y., Jin, N., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Cell Discovery on 10 June 2025 by Wang, Y., Ji, P., et al.
The discovery of broadly neutralizing antibodies (bNAbs) that target conserved epitopes on the HIV-1 envelope glycoprotein (Env) has garnered significant attention for its potential in the development of effective therapeutic and vaccine strategies. In this study, we isolated and characterized a CD4 binding site (CD4bs) antibody, FD22, from an elite neutralizer in China who had been infected with a clade B virus through contaminated blood plasma for 23 years. The heavy chain of FD22 was derived from a rarely reported IGHV3-30 germline gene and exhibited an exceptionally high degree of somatic hypermutation (SHM) (37%), along with a long and unique CDRH3 loop of 20-amino acids. FD22 exhibited potent and broad neutralizing activity, comparable to that of the well-known bNAb VRC01. It effectively neutralized 82% of a panel of 145 diverse HIV-1 pseudoviruses, including the two major circulating strains in China, CRF01_AE and CRF07_BC. FD22 bound strongly to HIV-1-infected cell lines, efficiently engaged FcγRIIIa receptors, triggered NK cell degranulation and the release of key cytokines such as IFN-γ and β-chemokines, and robustly induced antibody-dependent cellular cytotoxicity (ADCC) against HIV-1-infected target cells. Structural prediction for FD22 and the HIV Env SOSIP trimer performed by AlphaFold3, site-mutagenesis, and autologous virus reverse mutation assays revealed that the epitope of FD22 spans key CD4 binding site, including Loop D, the CD4 binding loop (CD4 BLP), and the V5 Loop. The unique long CDRH3 loop of FD22 interacts with the CD4 binding site through its negatively charged residue R102, distinguishing it from other CD4bs antibodies. Our findings provide valuable insights into the mechanisms of FD22 in viral neutralization and ADCC. The dual functionality of FD22 enhances its potential as a promising therapeutic antibody and offers new avenues for designing CD4bs-targeting vaccines with enhanced ADCC capabilities.
© 2025. The Author(s).
-
Immunology and Microbiology
In Respiratory Research on 4 June 2025 by Sompa, S. I., Ji, J., et al.
The use of electronic (e)-cigarettes in the long term has been associated with an increased risk of respiratory diseases. Dual use of e-cigarettes and traditional cigarettes may increase these risks even more due to the combined exposure effects of these products. The aim of this study was to investigate the local and systemic effects of e-cigarette use for more than one year and compare them with healthy non-smokers, cigarette smokers, and dual users.
The clinical study was conducted among 22 healthy non-smokers, 20 e-cigarette users, 20 cigarette smokers, and 20 dual users. Participants were matched with age and BMI, had normal baseline lung function, and had no allergies. Exhaled FeNO and bronchial responsiveness were assessed along with reactive oxygen species (ROS), toll-like receptor (TLR) expression, and inflammatory cytokines in blood and sputum.
Exhaled FeNO was higher in e-cigarette users (14 ppb, p = 0.04) and lower in cigarette smokers (9 ppb, p = 0.04) compared to healthy non-smokers (11 ppb). Bronchial responsiveness was increased in e-cigarette users (1.9 mg, p = 0.01) and cigarette smokers (1.9 mg, p = 0.01) compared to healthy non-smokers (2.9 mg). ROS in blood and sputum in e-cigarette users (p = 0.005 and p = 0.04) and dual users (p = 0.003 and p = 0.04) were increased. Also, TLR2 expression in blood granulocytes in all exposed groups (p = 0.001), TLR2 and TLR4 expression in sputum in e-cigarette users (p = 0.04 and p = 0.03) and dual users (p < 0.0001 and p = 0.004) were increased. Moreover, the percentage of IL13 and IFNγ cytokine-producing T cells in blood were increased in e-cigarette users (p = 0.0001 and p < 0.0001) and dual users (p = 0.001 and p < 0.0001).
Our research indicates that both local and systemic inflammatory responses, along with innate immune receptor activity, were significantly altered in e-cigarette users and dual users. Notably, these alterations were detected in e-cigarette users within a short timeframe of just 1 to 3 years of use.
Not applicable.
© 2025. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
In IScience on 21 March 2025 by Wei, Q., Foyn, H., et al.
FoxP3+ regulatory T cells (Tregs) are responsible for immune homeostasis by suppressing excessive anti-self-immunity. Tregs facilitate tumor growth by inhibiting anti-tumor immunity. Here, we explored the targeting of FoxP3 as a basis for new immunotherapies. In a high-throughput phenotypic screening of a drug repurposing library using human primary T cells, we identified quinacrine as a FoxP3 downregulator. In silico searches based on the structure of quinacrine, testing of sub-libraries of analogs in vitro, and validation identified a subset of 9-amino-acridines that selectively abrogated Treg suppressive functions. Mechanistically, these acridines interfered with the DNA-binding activity of FoxP3 and inhibited FoxP3-regulated downstream gene regulation. Release from Treg suppression by 9-amino-acridines increased anti-tumor immune responses both in cancer patient samples and in mice in a syngeneic tumor model. Our study highlights the feasibility of screening for small molecular inhibitors of FoxP3 as an approach to pursuing Treg-based immunotherapy.
© 2025 The Author(s).
-
Immunology and Microbiology
Novel B7-H3 CAR T cells show potent antitumor effects in glioblastoma: a preclinical study.
In Journal for Immunotherapy of Cancer on 25 January 2025 by Inthanachai, T., Boonkrai, C., et al.
B7 homolog 3 (B7-H3), an overexpressed antigen across multiple solid cancers, represents a promising target for CAR T cell therapy. This study investigated the expression of B7-H3 across various solid tumors and developed novel monoclonal antibodies (mAbs) targeting B7-H3 for CAR T cell therapy.
Expression of B7-H3 across various solid tumors was evaluated using RNA-seq data from TCGA, TARGET, and GTEx datasets and by flow cytometry staining. B7-H3-specific mAbs were developed by immunizing mice with human B7-H3, screening with ELISA, and analyzing kinetics with surface plasmon resonance. These mAbs were used to create second-generation CAR constructs, which were evaluated in vitro and in vivo for their antitumor function.
We identified four mAb clones from immunized mice, with three demonstrating high specificity and affinity. The second-generation B7-H3 CAR T cells derived from these mAbs exhibited robust cytotoxicity against B7-H3-positive targets and successfully infiltrated and eliminated tumor spheroids in vitro. In a xenograft mouse model of glioblastoma, these CAR T cells, particularly those derived from clone A2H4, eradicated the primary tumor, and effectively controlled rechallenge tumor, resulting in prolonged survival of the xenograft mice. In vivo T cell trafficking revealed high accumulation and persistence of A2H4-derived CAR T cells at the tumor site.
Our results provide novel B7-H3-targeted CAR T cells with high efficacy, paving the way for clinical translation of solid tumor treatment.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
-
Immunology and Microbiology
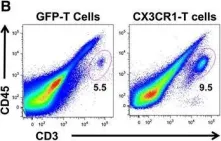
In J Immunother Cancer on 21 April 2016 by Siddiqui, I., Erreni, M., et al.
Fig.3.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Immunother Cancer by CiteAb, provided under a CC-BY license
Image 1 of 1