Adipose-derived stromal/stem cells (ASCs) are promising for the treatment of many diseases, including tissue injury or degeneration associated with serious disease and high morbidity. The extent of cell therapy effectiveness, however, may be limited by the lower survival of implanted cells in environments of tissue damage. Therefore, strategies to improve cell survival are important, such as by pretreating/preconditioning cells with a beneficial agent. We investigated the pretreatment of human ASCs (hASCs) with StemRegenin 1 (SR1), a purine derivative used in clinical protocols for in vitro hematopoietic stem/progenitor cell expansion. We pretreated hASCs with SR1 and analyzed the resulting cells (SR1-hASCs) as compared to non-treated cells (NT-hASCs). We noted that treatment with SR1 significantly increased the proliferation and migration of hASCs, as well as their secretion of paracrine factors of interest, and did not affect their cell differentiation capacity. Furthermore, when these SR1-hASCs were subsequently exposed to antimycin A, a mitochondrial respiratory chain inhibitor, they showed significantly higher antioxidative, anti-apoptotic, and pro-survival abilities as compared to NT-hASCs. Since oxidative stress and other harsh environments result from tissue damage, our results support that the preconditioning of hASCs with SR1 may enhance their protective, reparative, and regenerative, and thus therapeutic, efficacy.
© 2025 The Authors.
Product Citations: 151
In Molecular Therapy. Methods Clinical Development on 11 December 2025 by Zhao, J., Yu, B., et al.
-
Stem Cells and Developmental Biology
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 October 2025 by Da Ros, A., Benetton, M., et al.
Mesenchymal stromal cells (MSCs) are key components of the tumor microenvironment (TME), influencing leukemia progression through poorly understood mechanisms. Here, the bioelectrical properties of MSCs derived from pediatric acute myeloid leukemia (AML) patients (AML-MSCs) are investigated, identifying a significant depolarization of their resting voltage membrane potential (Vmem, -14.7 mV) compared to healthy MSCs (h-MSCs, -28.5 mV), accompanied by downregulation of Calcium channel, voltage-dependent, L type, alpha 1C subunit1.2 (CaV1.2) L-type calcium channel expression. AML-MSCs display increased spontaneous calcium oscillations, suggesting altered ion homeostasis. Notably, h-MSCs exposed to AML blasts undergo a similar Vmem depolarization (-11.8 mV) and CaV1.2 downregulation, indicating that leukemic cells actively reprogram MSCs. Functionally, Vmem depolarization in h-MSCs promotes a pro-leukemic phenotype, whereas hyperpolarization of AML-MSCs restores a normal behavior. CaV1.2 over-expression by lentiviral vectors in AML-MSCs shifts the Vmem toward hyperpolarization and partially reverses their leukemia-supportive properties, in part through CaV1.2 transfer via tunneling nanotubes. These findings reveal that AML blasts impose a bioelectrical signature on MSCs, modulating ion channel activity to sustain a leukemic niche. Targeting this electrical reprogramming through CaV1.2 restoration represents a potential strategy to re-establish homeostasis in the bone marrow microenvironment.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
In BMC Endocrine Disorders on 17 July 2025 by Agareva, M., Michurina, S., et al.
Adipose tissue secretome plays a crucial role in the mechanisms of metabolic diseases. Weight loss has a favourable effect on the adipose tissue secretome and prevents the development of type 2 diabetes mellitus (T2DM) and its complications. The most effective methods of glycaemic control are bariatric surgery (BS) and pharmacotherapy. The aim of our study is to evaluate changes in adipose tissue secretome after BS and semaglutide injections.
17 patients with T2DM were examined before and 6 months after BS or semaglutide therapy. The examination protocol included anthropometry, clinical biochemistry, insulin resistance evaluation and collection of subcutaneous adipose tissue biopsies. Adipose derived stem cells (ADSC) were isolated from biopsies according to a standard enzymatic protocol and differentiated into white and beige adipocytes. Adipogenesis and thermogenesis were assessed by confocal microscopy. Secretome of adipocytes and cytokines plasma levels were analyzed using a MILLIPLEX panel.
Following BS and semaglutide therapy, a decline in BMI, total fat content, HbA1c, and fasting blood glucose was observed. Insulin sensitivity increased only 6 months after BS. Semaglutide therapy resulted in the elevation of angiogenic and proinflammatory cytokines in adipocyte secretory profile. After BS we also detected the increase in proinflammatory cytokines both in adipocyte secretome and in plasma levels. However, the adipocyte secretome subsequent to bariatric surgery (BS) exhibited a reduced proinflammatory response in comparison to that observed following semaglutide therapy.
The effect of semaglutide injections directly on adipose tissue can change the function of ADSC, making them more angiogenic and adipogenic. A decrease in BMI, HbA1c and insulin resistance is achieved to a significant extent only after BS. BS-induced T2DM remission is related to lower pro-inflammatory secretion from adipocytes as compared to semaglutide. The regulation of inflammation in adipocytes may serve as a potential mechanism underlying BS-induced T2DM remission.
© 2025. The Author(s).
-
Endocrinology and Physiology
In Bio-protocol on 20 June 2025 by Singla, S., Khurana, S., et al.
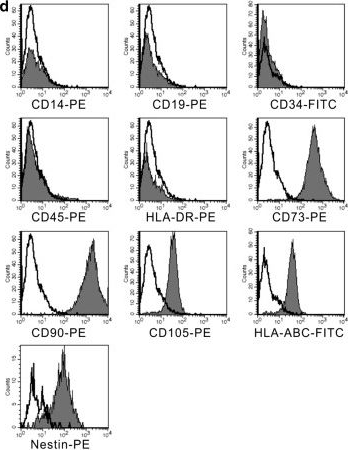
Cancer-associated mesenchymal stem cells (Ca-MSCs), an integral part of the tumor microenvironment, play a major role in modulating tumor progression; they have been reported to progress as well as inhibit various cancers, including cervical cancer. To understand the exact role of Ca-MSCs in tumor modulation, it is necessary to have an optimized protocol for Ca-MSCs isolation. This work demonstrates the isolation and expansion of a primary culture of cervical cancer-associated MSCs (CCa-MSCs) from the biopsy sample of cervical cancer patients using the explant culture technique. The isolated cells were characterized according to International Society for Cellular Therapy (ISCT) guidelines. Morphological analysis revealed that cells were adherent to the plastic surface and possessed spindle-shaped morphology. Flow cytometry analysis of the cells showed high expression (~98%) for MSC-specific cell surface markers (CD90, CD73, and CD105), negative expression (<0.5%) for endothelial cell marker (CD34) and hematopoietic cell marker (CD45), and negligible expression for HLA-DR, as recommended by ISCT. Further, trilineage differentiation potential analysis of the cells showed their osteogenic and chondrogenic potential and adipogenic differentiation. This standardized protocol will assist in the cultivation of CCa-MSCs and the study of their interactions with tumor cells and other components of the tumor microenvironment. This protocol may be utilized in the establishment of Ca-MSCs from other types of cancers as well. Key features • Isolation and expansion of cervical cancer-associated mesenchymal stem cells (CCa-MSCs) from patient biopsy sample. • Characterization of isolated CCa-MSCs for the presence of MSC-specific cell surface markers and trilineage differentiation potential. • CCa-MSCs can be employed to study the interactions with the tumor cells in the tumor microenvironment. Graphical overview.
©Copyright : © 2025 The Authors; This is an open access article under the CC BY-NC license.
-
Cancer Research
-
Stem Cells and Developmental Biology
In Materials Today. Bio on 1 June 2025 by Lin, Y., Yang, R. Y., et al.
Intervertebral disc degeneration (IDD) is characterized by oxidative-stress driven progressive apoptosis and senescence of nucleus pulposus mesenchymal stem cells (NP-MSCs). MOTS-c, a 16-amino acid peptide encoded by the mitochondrial 12S rRNA open reading frame, has emerged as a key regulator of cellular metabolism, oxidative stress, and senescence. This study investigated the therapeutic potential of MOTS-c in countering tert-butyl hydroperoxide (TBHP)-induced oxidative damage in NP-MSCs, and we developed a novel biomaterial strategy for IDD treatment.Key findings include.
MOTS-c significantly attenuated TBHP-induced NP-MSC apoptosis (Annexin V+/PI + cells reduced by 48 %, p < 0.001), senescence (SA-β-gal + cells decreased by 52 %, p < 0.005), and ROS overproduction (35 % reduction, p < 0.0001) via activation of the AMPK/SIRT1 pathway. Pharmacological inhibition of SIRT1 abolished these protective effects, confirming pathway specificity.
A sustained-release MOTS-c delivery system (RAD/RMOTS-c) was engineered by conjugating MOTS-c to the self-assembling RADA16-I peptide. The hydrogel exhibited a β-sheet-rich nanofibrous structure (fiber diameter: 362.6 nm), shear-thinning rheology (viscosity: 131-217 Pa s), and sustained peptide release over 7 days.
RAD/RMOTS-c enhanced NP-MSC viability (1.8-fold vs. control, p < 0.005) and extracellular matrix (ECM) synthesis, elevating collagen II/aggrecan expression (2.3-fold, p < 0.05) while suppressing collagen I (63 % reduction, p < 0.001).In Vivo Therapeutic Validation: In a rat IDD model, RAD/RMOTS-c injection preserved disc height (DHI%: 82.4 vs. 58.7 in IDD group, p < 0.001), restored T2-weighted MRI signals (1.5-fold increase, p < 0.001), and reduced histological degeneration scores by 44 % compared to untreated controls (p < 0.001).
This work (1) demonstrates the association between MOTS-c's anti-degenerative effects and AMPK/SIRT1 signaling in NP-MSCs and (2) pioneers a peptide-hydrogel hybrid system that synergistically combines mitochondrial protection with structural support for disc regeneration. The findings can advance IDD therapy toward biology-driven, minimally invasive solutions, aligning with the paradigm of functional biomaterials for degenerative diseases.
© 2025 The Authors.
-
Stem Cells and Developmental Biology
In Stem Cell Res Ther on 4 December 2014 by Wang, Y., Wu, H., et al.
Fig.2.D
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Stem Cell Research & Therapy by CiteAb, provided under a CC-BY license
Image 1 of 1
