Mesenchymal stem cells (MSCs) have been proposed to treat osteoarthritis (OA) for many years. However, clinical outcomes have been inconsistent due to biological variation between patients, differences in tissue source and preparation of the MSCs, and type of donor (e.g. allogenic versus autologous). Here, we test the hypothesis that inconsistent clinical outcomes are related to variations in the stemness and senescence of the injected autologous adipose-derived (AD) MSCs.
In the prospective randomized trial, 45 knee OA patients were divided into two groups: Group 1 (n = 22) patients treated with high tibial osteotomy (HTO) alone and Group 2 (n = 23) patients treated with HTO followed by intra-articular injection of autologous AD-MSCs (HTO + AD-MSCs). MRI and X-ray were performed pre-operation and 12 months post-operation. WOMAC and VAS score were collected four times, every 6 months over a 24-month follow-up. We observed the proliferation and stemness of AD-MSCs selected from the 5 patients showing the most improvement and from the 5 patients with the least improvement, and completed further in vitro experiments including beta-galactosidase activity, reactive oxygen species and bioinformatic analysis.
The results showed that patients treated with HTO + AD-MSCs had a significant reduction in knee OA severity as compared to patients treated with HTO alone. Moreover, we discovered that proliferation and colony forming efficiency of AD-MSCs selected from the 5 patients showing the most improvement performed significantly better than cells selected from the 5 patients with the least improvement. AD-MSCs from the patients with the most improvement also had lower amounts of senescent cells and intracellular reactive oxygen species.
Clinical outcomes of autologous AD-MSCs therapy in knee osteoarthritis are correlated with stem cell stemness and senescence. Our study highlights emerging opportunities and trends in precision medicine that could potentially improve autologous MSC-based therapies.
© 2024. The Author(s).
Product Citations: 17
In Journal of Translational Medicine on 18 November 2024 by Sun, H., Zhai, H., et al.
-
Stem Cells and Developmental Biology
In International Journal of Stem Cells on 30 November 2023 by Bashyal, N., Kim, M. G., et al.
Therapeutic efficacy of mesenchymal stem cells (MSCs) is determined by biodistribution and engraftment in vivo. Compared to intravenous infusion, biodistribution of locally transplanted MSCs are partially understood. Here, we performed a pharmacokinetics (PK) study of MSCs after local transplantation. We grafted human MSCs into the brains of immune-compromised nude mice. Then we extracted genomic DNA from brains, lungs, and livers after transplantation over a month. Using quantitative polymerase chain reaction with human Alu-specific primers, we analyzed biodistribution of the transplanted cells. To evaluate the role of residual immune response in the brain, MSCs expressing a cytosine deaminase (MSCs/CD) were used to ablate resident immune cells at the injection site. The majority of the Alu signals mostly remained at the injection site and decreased over a week, finally becoming undetectable after one month. Negligible signals were transiently detected in the lung and liver during the first week. Suppression of Iba1-positive microglia in the vicinity of the injection site using MSCs/CD prolonged the presence of the Alu signals. After local transplantation in xenograft animal models, human MSCs remain predominantly near the injection site for limited time without disseminating to other organs. Transplantation of human MSCs can locally elicit an immune response in immune compromised animals, and suppressing resident immune cells can prolong the presence of transplanted cells. Our study provides valuable insights into the in vivo fate of locally transplanted stem cells and a local delivery is effective to achieve desired dosages for neurological diseases.
-
Stem Cells and Developmental Biology
In Cell Reports on 25 July 2023 by Park, C. S., Yoshihara, H., et al.
The bone marrow microenvironment (BME) drives drug resistance in acute lymphoblastic leukemia (ALL) through leukemic cell interactions with bone marrow (BM) niches, but the underlying mechanisms remain unclear. Here, we show that the interaction between ALL and mesenchymal stem cells (MSCs) through integrin β1 induces an epithelial-mesenchymal transition (EMT)-like program in MSC-adherent ALL cells, resulting in drug resistance and enhanced survival. Moreover, single-cell RNA sequencing analysis of ALL-MSC co-culture identifies a hybrid cluster of MSC-adherent ALL cells expressing both B-ALL and MSC signature genes, orchestrated by a WNT/β-catenin-mediated EMT-like program. Blockade of interaction between β-catenin and CREB binding protein impairs the survival and drug resistance of MSC-adherent ALL cells in vitro and results in a reduction in leukemic burden in vivo. Targeting of this WNT/β-catenin-mediated EMT-like program is a potential therapeutic approach to overcome cell extrinsically acquired drug resistance in ALL.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Cancer Research
In Frontiers in Immunology on 11 February 2023 by Li, H., Xu, Y., et al.
The pathogenesis of autism spectrum disorder (ASD) is not well understood, especially in terms of immunity and inflammation, and there are currently no early diagnostic or treatment methods. In this study, we obtained six existing Gene Expression Omnibus transcriptome datasets from the blood of ASD patients. We performed functional enrichment analysis, PPI analysis, CIBERSORT algorithm, and Spearman correlation analysis, with a focus on expression profiling in hub genes and immune cells. We validated that monocytes and nonclassical monocytes were upregulated in the ASD group using peripheral blood (30 children with ASD and 30 age and sex-matched typically developing children) using flow cytometry. The receiver operating characteristic curves (PSMC4 and ALAS2) and analysis stratified by ASD severity (LIlRB1 and CD69) showed that they had predictive value using the "training" and verification groups. Three immune cell types - monocytes, M2 macrophages, and activated dendritic cells - had different degrees of correlation with 15 identified hub genes. In addition, we analyzed the miRNA-mRNA network and agents-gene interactions using miRNA databases (starBase and miRDB) and the DSigDB database. Two miRNAs (miR-342-3p and miR-1321) and 23 agents were linked with ASD. These findings suggest that dysregulation of the immune system may contribute to ASD development, especially dysregulation of monocytes and monocyte-derived cells. ASD-related hub genes may serve as potential predictors for ASD, and the potential ASD-related miRNAs and agents identified here may open up new strategies for the prevention and treatment of ASD.
Copyright © 2023 Li, Xu, Li, Zhang, Zhang, Li, Chen, Wang and Zhu.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
-
Neuroscience
In Molecules and Cells on 31 July 2022 by Bashyal, N., Lee, T. Y., et al.
Human mesenchymal stem cells (MSCs) are multipotent stem cells that have been intensively studied as therapeutic tools for a variety of disorders. To enhance the efficacy of MSCs, therapeutic genes are introduced using retroviral and lentiviral vectors. However, serious adverse events (SAEs) such as tumorigenesis can be induced by insertional mutagenesis. We generated lentiviral vectors encoding the wild-type herpes simplex virus thymidine kinase (HSV-TK) gene and a gene containing a point mutation that results in an alanine to histidine substitution at residue 168 (TK(A168H)) and transduced expression in MSCs (MSC-TK and MSC-TK(A168H)). Transduction of lentiviral vectors encoding the TK(A168H) mutant did not alter the proliferation capacity, mesodermal differentiation potential, or surface antigenicity of MSCs. The MSC-TK(A168H) cells were genetically stable, as shown by karyotyping. MSC-TK(A168H) responded to ganciclovir (GCV) with an half maximal inhibitory concentration (IC50) value 10-fold less than that of MSC-TK. Because MSC-TK(A168H) cells were found to be non-tumorigenic, a U87-TK(A168H) subcutaneous tumor was used as a SAE-like condition and we evaluated the effect of valganciclovir (vGCV), an oral prodrug for GCV. U87-TK(A168H) tumors were more efficiently ablated by 200 mg/kg vGCV than U87-TK tumors. These results indicate that MSC-TK(A168H) cells appear to be pre-clinically safe for therapeutic use. We propose that genetic modification with HSV-TK(A168H) makes allogeneic MSC-based ex vivo therapy safer by eliminating transplanted cells during SAEs such as uncontrolled cell proliferation.
-
Genetics
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
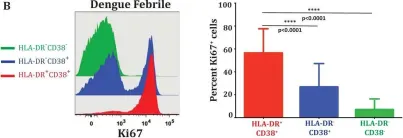
In J Virol on 15 December 2016 by Chandele, A., Sewatanon, J., et al.
Fig.1.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Virol by CiteAb, provided under a CC-BY license
Image 1 of 1