Breast cancer (BC) is commonly labeled a "cold tumor" due to its dense population of immunosuppressive cells, particularly M2-like macrophages, which contribute to its resistance to therapy. Thus, there is a pressing need to shift the macrophage polarization towards M1 and revitalize the tumor immune microenvironment (TIME) to improve BC prognosis. In this study, we leveraged published RNA-sequencing data and performed multiplex immunohistochemistry on clinical specimens to identify NR4A3 as a promising biomarker for favorable outcomes in BC. High NR4A3 expression correlates with an inflamed TIME, characterized by heightened T-cell infiltration and activation. NR4A3 was preferentially expressed in macrophages and fostered M1-like macrophage polarization through direct binding to p65, thereby enhancing NF-κB transcriptional activity. Overexpression of Nr4a3 in tumor-infiltrating macrophages significantly inhibited the growth of E0771 tumors in a syngeneic mouse model, accompanied by increased T-cell infiltration and elevated production of functional cytokines. Conversely, suppression of Nr4a3 in macrophages compromised T-cell recruitment and diminished their anti-tumor capabilities. Consistent with these findings, co-culture experiments involving human T cells and NR4A3-overexpressing THP1 cells further demonstrated enhanced T-cell functionality. Collectively, our findings uncover a novel role for NR4A3 in macrophage polarization and TIME remodeling, offering a potential biomarker for favorable BC prognosis and a therapeutic target to enhance immunotherapy efficacy.
© 2025. The Author(s).
Product Citations: 13
In NPJ Breast Cancer on 7 July 2025 by Qian, Y. Y., Jin, N., et al.
-
Cancer Research
-
Immunology and Microbiology
In Cells on 30 March 2025 by Boros-Rausch, A., Dorogin, A., et al.
The uterine smooth muscle (myometrium) is an immunomodulatory tissue capable of secreting multiple chemokines during pregnancy. We propose that before term labor, chemokines secreted as a result of mechanical stretch of the uterine walls by the growing fetus(es) induce infiltration of maternal monocytes into myometrium, drive their differentiation into macrophages, and induce pro-inflammatory (M1) polarization, leading to labor contractions. This study used high-throughput proteomic mass-spectrometry to investigate the underlying mechanisms and explored the therapeutic potential of a broad-spectrum chemokine inhibitor (BSCI, FX125L) in modulating these effects. Primary myocytes isolated from the myometrium of term pregnant women were subjected in vitro to static mechanical stretch. Proteomic analysis of stretched myocyte-conditioned media (CM) identified significant upregulation of chemokine-related pathways and ECM degradation proteins. CM induced in vitro differentiation of human monocytes to macrophages and polarization into an M1-like phenotype characterized by elevated ROS production. BSCI treatment altered the myocyte secretome, increasing tissue-remodeling and anti-inflammatory proteins, Annexin A1 and TGF-β. BSCI-treated myocyte secretions induced Annexin A1 expression in macrophages and enhanced their phagocytic activity. We conclude that factors secreted by mechanically stretched myocytes induce pro-inflammatory M1 macrophage polarization, while BSCI modulates myocyte secretome, which reprograms macrophages to a homeostatic M2-like phenotype, thus reducing inflammation. When treated with BSCI, M2-polarized macrophages reduced myocyte-driven collagen gel contraction, whereas M1 macrophages enhanced it. This study reveals novel insights into the myocyte-macrophage interaction and identifies BSCI as a promising drug to modulate myometrial activity. We suggest that uterine macrophages may represent a therapeutic target for preventing preterm labor in women.
-
Cell Biology
-
Immunology and Microbiology
Preprint on Research Square on 7 November 2024 by Zhang, X., Yang, J., et al.
Abstract Objective The long non-coding RNA (lncRNA) PVT1 plays a significant role in regulating the development and progression of various cancers. However, its clinical relevance in triple-negative breast cancer (TNBC) and its immunoregulatory mechanisms in TNBC remain largely unexplored. Methods An orthotopic TNBC mouse model was established, and single-cell RNA sequencing was performed on tumor tissues to examine macrophage populations. Bulk RNA sequencing, differential expression analysis, and Weighted Gene Co-expression Network Analysis were integrated to identify key factors of interest. Experiments using the co-culture si-PVT1-transduced oe-PPARγ TNBC cells with macrophages were conducted to observe their effects on TNBC cell growth and on M1/M2 marker expression both in vivo and in vitro. Additionally, the interactions of PVT1, NOP56, and E2F1 and their influence on PPARγ transcription were analyzed using RNA/DNA immunoprecipitation, ChIP-qPCR, and luciferase reporter assays. Results Macrophage reprogramming occurred in the TNBC tissues of mice, characterized by a significant accumulation of M2-type macrophages in tumor tissues. Both PVT1 and PPARγ play pivotal roles in this reprogramming. PVT1 knockdown (KD) suppressed the expression of PPARγ and M2 macrophage markers, while oe-PPARγ partially restored M2 marker expression. In vitro, PVT1 enhances TNBC cell proliferation, invasion, and metastasis through PPARγ. Similarly, in vivo, PVT1 promotes TNBC tumor growth and M2 marker expression via PPARγ. Mechanistically, PVT1 functions as a scaffold to recruit NOP56 and E2F1, forming a PVT1–NOP56–E2F1 complex that facilitates the transcriptional upregulation of PPARγ. Conclusion LncRNA PVT1 significantly affects macrophage polarization and TNBC progression by regulating PPARγ transcription. These findings suggest novel molecular targets for TNBC therapy development.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
S100A9 and HMGB1 orchestrate MDSC-mediated immunosuppression in melanoma through TLR4 signaling.
In Journal for Immunotherapy of Cancer on 11 September 2024 by Ozbay Kurt, F. G., Cicortas, B. A., et al.
Immunotherapies for malignant melanoma are challenged by the resistance developed in a significant proportion of patients. Myeloid-derived suppressor cells (MDSC), with their ability to inhibit antitumor T-cell responses, are a major contributor to immunosuppression and resistance to immune checkpoint therapies in melanoma. Damage-associated molecular patterns S100A8, S100A9, and HMGB1, acting as toll like receptor 4 (TLR4) and receptor for advanced glycation endproducts (RAGE) ligands, are highly expressed in the tumor microenvironment and drive MDSC activation. However, the role of TLR4 and RAGE signaling in the acquisition of MDSC immunosuppressive properties remains to be better defined. Our study investigates how the signaling via TLR4 and RAGE as well as their ligands S100A9 and HMGB1, shape MDSC-mediated immunosuppression in melanoma.
MDSC were isolated from the peripheral blood of patients with advanced melanoma or generated in vitro from healthy donor-derived monocytes. Monocytes were treated with S100A9 or HMGB1 for 72 hours. The immunosuppressive capacity of treated monocytes was assessed in the inhibition of T-cell proliferation assay in the presence or absence of TLR4 and RAGE inhibitors. Plasma levels of S100A8/9 and HMGB1 were quantified by ELISA. Single-cell RNA sequencing (scRNA-seq) was performed on monocytes from patients with melanoma and healthy donors.
We showed that exposure to S100A9 and HMGB1 converted healthy donor-derived monocytes into MDSC through TLR4 signaling. Our scRNA-seq data revealed in patient monocytes enriched inflammatory genes, including S100 and those involved in NF-κB and TLR4 signaling, and a reduced major histocompatibility complex II gene expression. Furthermore, elevated plasma S100A8/9 levels correlated with shorter progression-free survival in patients with melanoma.
These findings highlight the critical role of TLR4 and, to a lesser extent, RAGE signaling in the conversion of monocytes into MDSC-like cells, underscore the potential of targeting S100A9 to prevent this conversion, and highlight the prognostic value of S100A8/9 as a plasma biomarker in melanoma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
Homo sapiens (Human)
-
Cancer Research
In IScience on 16 June 2023 by Ji, D., Jia, J., et al.
Mucinous colorectal adenocarcinoma (MC) is less likely to respond to chemotherapy and is associated with poorer prognosis compared with non-MC (NMC). Fibroblast activation protein (FAP) was found and validated to be upregulated in MC patients and was negatively correlated with prognosis and therapeutic outcomes in colorectal cancer (CRC) patients who were treated with adjuvant chemotherapy. Overexpression of FAP promoted CRC cell growth, invasion and metastasis, and enhanced chemoresistance. Myosin phosphatase Rho-interacting protein (MPRIP) was identified as a direct interacting protein of FAP. FAP may influence the efficiency of chemotherapy and prognosis by promoting the crucial functions of CRC and inducing tumor-associated macrophages (TAMs) recruitment and M2 polarization through regulating theRas Homolog Family Member/Hippo/Yes-associated protein (Rho/Hippo/YAP) signaling pathway. Knockdown of FAP could reverse tumorigenicity and chemoresistance in CRC cells. Thus, FAP may serve as a marker for prognosis and therapeutic outcome, as well as a potential therapeutic target to overcome chemoresistance in MC patients.
© 2023 The Authors.
-
Cancer Research
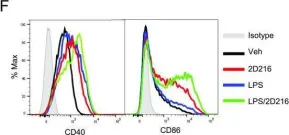
In ACS Chem Biol on 21 January 2022 by Saito, T., Shukla, N. M., et al.
Fig.2.F

-
FC/FACS
-
Collected and cropped from ACS Chem Biol by CiteAb, provided under a CC-BY license
Image 1 of 2
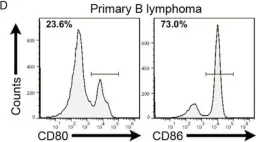
In Front Immunol on 20 April 2021 by Lin, S., Cheng, L., et al.
Fig.2.D

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2