Phosphoglycerate dehydrogenase (PHGDH) is the rate-limiting enzyme in the de novo Serine synthesis pathway (SSP), a highly regulated pathway overexpressed in several tumors. Specifically, PHGDH expression is dynamically regulated during different stages of tumor progression, promoting cancer aggressiveness. Previously, we demonstrated that high Serine (Ser) availability, obtained by increased exogenous uptake or increased PHGDH expression, supports 5-Fluorouracil (5-FU) resistance in colorectal cancer (CRC). Beyond its metabolic role in sustaining Ser biosynthesis, different "non-enzymatic roles" for PHGDH have recently been identified. The present study aims to investigate non-enzymatic mechanisms through which PHGDH regulates 5-FU response in CRC.
Overexpression and gene silencing approaches have been used to modulate PHGDH expression in human CRC cell lines to investigate the role of this enzyme in 5-FU cellular response. Identified mechanisms have been validated in selected 5-FU resistant cell lines, CRC patients-derived tumor tissue samples, and patients-derived 3D organoids. Transcriptomic analysis was performed on wild-type and PHGDH-silenced cell lines, allowing the identification of pathways responsible for PHGDH-mediated 5-FU resistance. The relevance of identified genes was validated in vitro and in vivo in a CRC xenograft model.
PHGDH expression is highly variable among CRC tissues and patient-derived 3D organoids. A retrospective analysis of CRC patients highlighted a correlation between PHGDH expression and therapy response. Coherently, the modulation of PHGDH expression by gene silencing/overexpression affects 5-FU sensitivity in CRC cell lines. Transcriptomic analysis on CRC cell lines stably silenced for PHGDH evidenced down regulation in Hedgehog (HH) pathway. Accordingly, in vitro and in vivo studies demonstrated that the combined treatment of 5-FU and HH pathway inhibitors strongly hinders CRC cell survival and tumor growth in CRC xenograft models.
PHGDH sustains 5-FU resistance in CRC by mediating the upregulation of the HH signaling; targeting the here identified PHGDH-HH axis increases 5-FU susceptibility in different CRC models suggesting the 5-FU/HH-inhibitors combinatorial therapeutic strategy as a valid approach to counteract drug resistance in CRC.
© 2025. The Author(s).
Product Citations: 51
PHGDH drives 5-FU chemoresistance in colorectal cancer through the Hedgehog signaling.
In Journal of Experimental & Clinical Cancer Research : CR on 10 July 2025 by Mancini, C., Lori, G., et al.
-
Cancer Research
In International Journal of Stem Cells on 30 May 2025 by Lee, N. K., Na, D. L., et al.
Mesenchymal stem cells (MSCs) are frequently used for therapeutic applications in both pre-clinical and clinical settings owing to their capacity for immune modulation and neuroprotective effects. However, transient fever is commonly observed as an adverse event following MSC injection in patients with Alzheimer's disease (AD). In this study, we investigated the potential impact of immunosuppressants such as dexamethasone and tacrolimus on altering the characteristics of human mesenchymal stem cells (hMSCs). Additionally, we examined whether these immunosuppressants affect the persistence of hMSCs or the immune response upon their administration into the brain parenchyma of AD mice. The exposure of hMSCs to high concentrations of dexamethasone and tacrolimus in vitro did not significantly alter the characteristics of hMSCs. The expression of genes related to innate immune responses, such as Irak1, Irf3, Nod1, and Ifnar1, was significantly downregulated by the additional administration of dexamethasone and tacrolimus to the brain parenchyma of AD mice. However, hMSC persistence in the AD mouse brain was not affected. The results of this study support the use of immunosuppressants to mitigate fever during stem cell therapy in patients with AD.
-
FC/FACS
-
Stem Cells and Developmental Biology
In Gels (Basel, Switzerland) on 18 March 2025 by De Mori, A., Aydin, N., et al.
Mesenchymal stem cells (MSCs) can differentiate into chondrocytes provided with the appropriate environmental cues. In this study, we loaded human adipose-derived stem cells (hAdMSCs) into collagen/alginate hydrogels, which have been shown to induce chondrogenesis in ovine bone marrow stem cells without the use of any exogenous chondrogenic growth factors. We examined the influence of hydrogel stiffness (5.75 and 6.85 kPa) and cell seeding density (1, 2, 4, and 16 × 106 cells/mL) on the chondrogenic induction of hAdMSCs, without exogenous differentiation growth factors. Over time, the behaviour of the hAdMSCs in the scaffolds was investigated by analysing the amount of DNA; their morphology; their cell viability; the expression of chondrogenic genes (RT-qPCR); and the deposition of collagen I, collagen II, and aggrecan. The results showed that all scaffolds supported the acquisition of a rounded morphology and the formation of cell aggregates, which were larger with higher cell seeding densities. Furthermore, the cells were viable within the hydrogels throughout the experiment, indicating that high cell density did not have a detrimental effect on viability. All the conditions supported the upregulation of chondrogenic genes (SOX9, COL2A1, SOX5, and ACAN). By comparison, only the highest cell seeding density (16 × 106 cells/mL) promoted a superior extracellular matrix deposition composed of collagen II and aggrecan with limited production of collagen I. These molecules were deposited in the pericellular space. Furthermore, no histological difference was noted between the two stiffnesses.
-
Stem Cells and Developmental Biology
In PLoS ONE on 23 October 2024 by Kilercik, M., Ozgur, E., et al.
Prostate cancer (PCa) is the second most common cancer among men and the fifth leading cause of cancer death. Circulating tumor cell (CTC) enumeration and characterisation in PCa has been shown to provide valuable information on prognosis of disease, therapy management and detection of resistance. Here, Cellsway's microfluidic platform for high-throughput enrichment of intact CTC populations was used to isolate CTCs from the blood of 20 localised PCa patients and 10 healthy donor samples to evaluate the clinical performance of the technology. To enumerate and characterise CTCs, a multi-parameter flow cytometry analysis was performed on the enriched CTC suspension using CTC-specific biomarkers. CTCs were detected in 17 of 20 patient samples, which corresponds to 85% CTC positivity. The median CTC count per 7.5 ml blood was 2 (1-9). In 80% of patients (n = 16), the number of CTCs ranged from 1 to 5, and in 5% of patients (n = 1) the number of CTCs was above 5. No CTCs were observed in the blood samples of 10 healthy volunteers, demonstrating the high specificity and low risk of false positives of the technology.
Copyright: © 2024 Kilercik et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
FC/FACS
-
Cancer Research
In ENeuro on 1 September 2024 by Lendemeijer, B., Unkel, M., et al.
Astrocytes are essential for the formation and maintenance of neural networks. However, a major technical challenge for investigating astrocyte function and disease-related pathophysiology has been the limited ability to obtain functional human astrocytes. Despite recent advances in human pluripotent stem cell (hPSC) techniques, primary rodent astrocytes remain the gold standard in coculture with human neurons. We demonstrate that a combination of leukemia inhibitory factor (LIF) and bone morphogenetic protein-4 (BMP4) directs hPSC-derived neural precursor cells to a highly pure population of astroglia in 28 d. Using single-cell RNA sequencing, we confirm the astroglial identity of these cells and highlight profound transcriptional adaptations in cocultured hPSC-derived astrocytes and neurons, consistent with their further maturation. In coculture with human neurons, multielectrode array recordings revealed robust network activity of human neurons in a coculture with hPSC-derived or rat astrocytes [3.63 ± 0.44 min-1 (hPSC-derived), 2.86 ± 0.64 min-1 (rat); p = 0.19]. In comparison, we found increased spike frequency within network bursts of human neurons cocultured with hPSC-derived astrocytes [56.31 ± 8.56 Hz (hPSC-derived), 24.77 ± 4.04 Hz (rat); p < 0.01], and whole-cell patch-clamp recordings revealed an increase of postsynaptic currents [2.76 ± 0.39 Hz (hPSC-derived), 1.07 ± 0.14 Hz (rat); p < 0.001], consistent with a corresponding increase in synapse density [14.90 ± 1.27/100 μm2 (hPSC-derived), 8.39 ± 0.63/100 μm2 (rat); p < 0.001]. Taken together, we show that hPSC-derived astrocytes compare favorably with rat astrocytes in supporting human neural network activity and maturation, providing a fully human platform for investigating astrocyte function and neuronal-glial interactions.
Copyright © 2024 Lendemeijer et al.
-
Neuroscience
-
Stem Cells and Developmental Biology
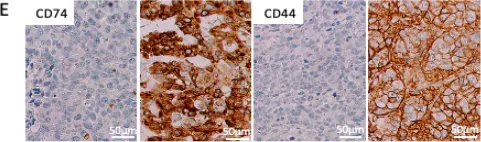
In Int J Mol Sci on 29 June 2021 by Loreth, D., Schuette, M., et al.
Fig.2.E

-
IHC
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 2
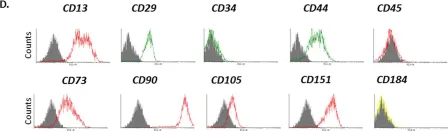
In NPJ Microgravity on 18 November 2017 by Weiss, W. M., Mulet-Sierra, A., et al.
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from NPJ Microgravity by CiteAb, provided under a CC-BY license
Image 1 of 2