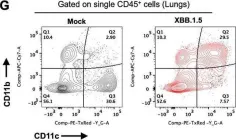
Owing to their continuous evolution, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) display disparate pathogenicity in mouse models. Omicron and its sublineages have been dominant worldwide. Compared to pre-Omicron VOCs, early Omicron subvariants reportedly cause attenuated disease in human ACE-2-expressing mice (K18-hACE-2). In late 2022, the frequency of Omicron subvariant XBB.1.5 rapidly increased and it progressively replaced other circulating strains. The emergence of new strains requires current SARS-CoV-2 clinical animal model re-evaluation. In this study, we aim to characterize XBB.1.5 pathogenesis in K18-hACE-2. Herein, we demonstrated that XBB.1.5 infection is associated with significant weight loss, severe lung pathology, and substantial mortality. Intranasal XBB.1.5 infection resulted in 100% mortality in K18-hACE2 mice. High virus titers were detected in the lungs on days 3 and 5 after infection. Moreover, XBB.1.5 productively infected the cells within the nasal turbinate, olfactory bulb, intestines, and kidneys. In addition, in a subset of infected mice, we detected high virus titers in the brain. Consistently, we detected high viral antigen expression in the lungs. Furthermore, we observed severe lung injury hallmarks (e.g., immune cell infiltration, perivascular cuffing, and alveolar consolidation). Using immunofluorescence labeling and cytometric analysis, we revealed that XBB.1.5 infection leads to CD45+ cell influx into the lung parenchyma. We further demonstrated that most immune infiltrates are CD11b+ CD11c+ dendritic cells. Additionally, we detected significant induction of proinflammatory cytokines and chemokines in infected lungs. Taken together, our data show that Omicron subvariant XBB.1.5 is highly pathogenic in K18-hACE2 mice.
Copyright © 2024 Elsharkawy, Stone, Guglani, Patterson, Ge, Dim, Miano and Kumar.
Product Citations: 26
Omicron XBB.1.5 subvariant causes severe pulmonary disease in K18-hACE-2 mice.
In Frontiers in Microbiology on 17 October 2024 by Elsharkawy, A., Stone, S., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
In Nature Communications on 31 August 2023 by Ortega-Ferreira, C., Soret, P., et al.
Systemic sclerosis (SSc) is an autoimmune, inflammatory and fibrotic disease with limited treatment options. Developing new therapies is therefore crucial to address patient needs. To this end, we focused on galectin-3 (Gal-3), a lectin known to be associated with several pathological processes seen in SSc. Using RNA sequencing of whole-blood samples in a cross-sectional cohort of 249 patients with SSc, Gal-3 and its interactants defined a strong transcriptomic fingerprint associated with disease severity, pulmonary and cardiac malfunctions, neutrophilia and lymphopenia. We developed new Gal-3 neutralizing monoclonal antibodies (mAb), which were then evaluated in a mouse model of hypochlorous acid (HOCl)-induced SSc. We show that two of these antibodies, D11 and E07, reduced pathological skin thickening, lung and skin collagen deposition, pulmonary macrophage content, and plasma interleukin-5 and -6 levels. Moreover, E07 changed the transcriptional profiles of HOCl-treated mice, resulting in a gene expression pattern that resembled that of control mice. Similarly, pathological pathways engaged in patients with SSc were counteracted by E07 in mice. Collectively, these findings demonstrate the translational potential of Gal-3 blockade as a therapeutic option for SSc.
© 2023. Springer Nature Limited.
Preprint on BioRxiv : the Preprint Server for Biology on 23 June 2023 by Mercado-Evans, V., Mejia, M. E., et al.
ABSTRACT Group B Streptococcus (GBS) is a pervasive perinatal pathogen, yet factors driving GBS dissemination in utero are poorly defined. Gestational diabetes mellitus (GDM), a complication marked by dysregulated immunity and maternal microbial dysbiosis, increases risk for GBS perinatal disease. We interrogated host-pathogen dynamics in a novel murine GDM model of GBS colonization and perinatal transmission. GDM mice had greater GBS in utero dissemination and subsequently worse neonatal outcomes. Dual-RNA sequencing revealed differential GBS adaptation to the GDM reproductive tract, including a putative glycosyltransferase ( yfhO ), and altered host responses. GDM disruption of immunity included reduced uterine natural killer cell activation, impaired recruitment to placentae, and altered vaginal cytokines. Lastly, we observed distinct vaginal microbial taxa associated with GDM status and GBS invasive disease status. Our translational model of GBS perinatal transmission in GDM hosts recapitulates several clinical aspects and enables discovery of host and bacterial drivers of GBS perinatal disease.
-
Immunology and Microbiology
Microbiota-derived acetate enhances host antiviral response via NLRP3.
In Nature Communications on 6 February 2023 by Niu, J., Cui, M., et al.
Pathogenic viral infections represent a major challenge to human health. Host immune responses to respiratory viruses are closely associated with microbiome and metabolism via the gut-lung axis. It has been known that host defense against influenza A virus (IAV) involves activation of the NLRP3 inflammasome, however, mechanisms behind the protective function of NLRP3 are not fully known. Here we show that an isolated bacterial strain, Bifidobacterium pseudolongum NjM1, enriched in the gut microbiota of Nlrp3-/- mice, protects wild-type but not Nlrp3 deficient mice against IAV infection. This effect depends on the enhanced production of type I interferon (IFN-I) mediated by NjM1-derived acetate. Application of exogenous acetate reproduces the protective effect of NjM1. Mechanistically, NLRP3 bridges GPR43 and MAVS, and promotes the oligomerization and signalling of MAVS; while acetate enhances MAVS aggregation upon GPR43 engagement, leading to elevated IFN-I production. Thus, our data support a model of NLRP3 mediating enhanced induction of IFN-I via acetate-producing bacterium and suggest that the acetate-GPR43-NLRP3-MAVS-IFN-I signalling axis is a potential therapeutic target against respiratory viral infections.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
Piperlongumine alleviates corneal allograft rejection via suppressing angiogenesis and inflammation.
In Frontiers in Immunology on 3 January 2023 by Fan, X., Qiu, J., et al.
Neovascularization and inflammatory response are two essential features of corneal allograft rejection. Here, we investigated the impact of Piperlongumine (PL) on alleviating corneal allograft rejection, primarily focusing on pathological angiogenesis and inflammation.
A murine corneal allograft transplantation model was utilized to investigate the role of PL in preventing corneal allograft rejection. PL (10 mg/kg) or vehicle was intraperitoneally injected daily into BALB/c recipients from day -3 to day 14. The clinical signs of the corneal grafts were monitored for 30 days. Corneal neovascularization and inflammatory cell infiltration were detected by immunofluorescence staining and immunohistochemistry. The proportion of CD4+ T cells and macrophages in the draining lymph nodes (DLNs) was examined by flow cytometry. In vitro, HUVECs were cultured under hypoxia or incubated with TNF-α to mimic the hypoxic and inflammatory microenvironment favoring neovascularization in corneal allograft rejection. Multiple angiogenic processes including proliferation, migration, invasion and tube formation of HUVECs in hypoxia with or without PL treatment were routinely evaluated. The influence of PL treatment on TNF-α-induced pro-inflammation in HUVECs was investigated by real-time PCR and ELISA.
In vivo, PL treatment effectively attenuated corneal allograft rejection, paralleled by coincident suppression of neovascularization and alleviation of inflammatory response. In vitro, PL distinctively inhibited hypoxia-induced angiogenic processes in HUVECs. Two key players in hypoxia-induced angiogenesis, HIF-1α and VEGF-A were significantly suppressed by PL treatment. Also, TNF-α-induced pro-inflammation in HUVECs was hampered by PL treatment, along with a pronounced reduction in ICAM-1, VCAM-1, CCL2, and CXCL5 expression.
The current study demonstrated that PL could exhibit both anti-angiogenic and anti-inflammatory effects in preventing corneal allograft rejection, highlighting the potential therapeutic applications of PL in clinical strategy.
Copyright © 2022 Fan, Qiu, Yuan, Zhang and Xu.
-
FC/FACS
-
Immunology and Microbiology
In Front Microbiol on 17 October 2024 by Elsharkawy, A., Stone, S., et al.
Fig.3.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Microbiol by CiteAb, provided under a CC-BY license
Image 1 of 1