Iron plays a major role in the deterioration of β-thalassemia. Indeed, the high levels of transferrin saturation and iron delivered to erythroid progenitors are associated with production of α-globin precipitates that negatively affect erythropoiesis. Matriptase-2/TMPRSS6, a membrane-bound serine protease expressed in hepatocytes, negatively modulates hepcidin production and thus is a key target to prevent iron overload in β-thalassemia. To address safety concerns raised by the suppression of Tmprss6 by antisense oligonucleotides or small interfering RNA, we tested a fully human anti-matriptase-2 antibody, RLYB331, which blocks the protease activity of matriptase-2. When administered weekly to Hbbth3/+ mice, RLYB331 induced hepcidin expression, reduced iron loading, prevented the formation of toxic α-chain/heme aggregates, reduced ros oxygen species formation, and improved reticulocytosis and splenomegaly. To increase the effectiveness of RLYB331 in β-thalassemia treatment even further, we administered RLYB331 in combination with RAP-536L, a ligand-trapping protein that contains the extracellular domain of activin receptor type IIB and alleviates anemia by promoting differentiation of late-stage erythroid precursors. RAP-536L alone did not prevent iron overload but significantly reduced apoptosis in the erythroid populations of the bone marrow, normalized red blood cell counts, and improved hemoglobin and hematocrit levels. Interestingly, the association of RLYB331 with RAP-536L entirely reversed the β-thalassemia phenotype in Hbbth3/+ mice and simultaneously corrected iron overload, ineffective erythropoiesis, splenomegaly, and hematological parameters, suggesting that a multifunctional molecule consisting of the fusion of RLYB331 with luspatercept (human version of RAP-536L) would allow administration of a single medication addressing simultaneously the different pathophysiological aspects of β-thalassemia.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Product Citations: 24
In Blood Advances on 23 April 2024 by Wake, M. S., Palin, A., et al.
-
Mus musculus (House mouse)
Cell state dependent effects of Bmal1 on melanoma immunity and tumorigenicity.
In Nature Communications on 20 January 2024 by Zhang, X., Pant, S. M., et al.
The circadian clock regulator Bmal1 modulates tumorigenesis, but its reported effects are inconsistent. Here, we show that Bmal1 has a context-dependent role in mouse melanoma tumor growth. Loss of Bmal1 in YUMM2.1 or B16-F10 melanoma cells eliminates clock function and diminishes hypoxic gene expression and tumorigenesis, which could be rescued by ectopic expression of HIF1α in YUMM2.1 cells. By contrast, over-expressed wild-type or a transcriptionally inactive mutant Bmal1 non-canonically sequester myosin heavy chain 9 (Myh9) to increase MRTF-SRF activity and AP-1 transcriptional signature, and shift YUMM2.1 cells from a Sox10high to a Sox9high immune resistant, mesenchymal cell state that is found in human melanomas. Our work describes a link between Bmal1, Myh9, mouse melanoma cell plasticity, and tumor immunity. This connection may underlie cancer therapeutic resistance and underpin the link between the circadian clock, MRTF-SRF and the cytoskeleton.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
Allergen-induced CD11c + dendritic cell pyroptosis aggravates allergic rhinitis.
In Cell Communication and Signaling : CCS on 10 October 2023 by Qiao, Y. L., Zhu, M. W., et al.
Pyroptosis is crucial for controlling various immune cells. However, the role of allergen-induced CD11c + dendritic cell (DC) pyroptosis in allergic rhinitis (AR) remains unclear.
Mice were grouped into the control group, AR group and necrosulfonamide-treated AR group (AR + NSA group). The allergic symptom scores, OVA-sIgE titres, serum IL-1β/IL-18 levels, histopathological characteristics and T-helper cell-related cytokines were evaluated. CD11c/GSDMD-N-positive cells were examined by immunofluorescence analysis. Murine CD11c + bone marrow-derived DCs (BMDCs) were induced in vitro, stimulated with OVA/HDM, treated with necrosulfonamide (NSA), and further cocultured with lymphocytes to assess BMDC function. An adoptive transfer murine model was used to study the role of BMDC pyroptosis in allergic rhinitis.
Inhibiting GSDMD-N-mediated pyroptosis markedly protected against Th1/Th2/Th17 imbalance and alleviated inflammatory responses in the AR model. GSDMD-N was mainly coexpressed with CD11c (a DC marker) in AR mice. In vitro, OVA/HDM stimulation increased pyroptotic morphological abnormalities and increased the expression of pyroptosis-related proteins in a dose-dependent manner; moreover, inhibiting pyroptosis significantly decreased pyroptotic morphology and NLRP3, C-Caspase1 and GSDMD-N expression. In addition, OVA-induced BMDC pyroptosis affected CD4 + T-cell differentiation and related cytokine levels, leading to Th1/Th2/Th17 cell imbalance. However, the Th1/Th2/Th17 cell immune imbalance was significantly reversed by NSA. Adoptive transfer of OVA-loaded BMDCs promoted allergic inflammation, while the administration of NSA to OVA-loaded BMDCs significantly reduced AR inflammation.
Allergen-induced dendritic cell pyroptosis promotes the development of allergic rhinitis through GSDMD-N-mediated pyroptosis, which provides a clue to allergic disease interventions. Video Abstract.
© 2023. BioMed Central Ltd., part of Springer Nature.
-
FC/FACS
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 12 August 2023 by Fein, D. E., Traugh, N., et al.
Fibroblasts are a major cell type within breast microenvironment which play key roles in tissue remodeling during the processes of normal development, injury, and malignancy. During wound healing and tumorigenesis, fibroblasts facilitate production and degradation of the extracellular matrix and produce inflammatory mediators which act as immune regulators. Domain Discoidin Receptor 1 (DDR1) is a cell surface tyrosine kinase receptor expressed by epithelial and stromal cells which is activated by collagen. In the breast, DDR1 expression and activity has been implicated in the development of fibrosis as well as chemotherapy resistance. We set out to examine whether selective inhibition of DDR1 would modulate fibroblast immunomodulatory function to generate an immune-permissive breast microenvironment and reduce stromal desmoplasia. In vivo, DDR1 inhibition resulted in mammary fibroblast tissue remodeling, reduced collagen deposition, and changes in immunomodulatory cytokine expression. Furthermore, DDR1 inhibition was associated with increased CD45.2+ immune cell infiltration and reduced Ly6G+/Ly6C− neutrophil infiltration. Mechanistically, we developed an ex-vivo 3D collagen hydrogel model of desmoplasia to study the effects of DDR1 inhibition on the expression of immune modulating factors and fibroblast functions and features. We found that DDR1 regulates the expression and secretion of key immunomodulatory cytokines (IL-6, IL-8, and MCP-1). Collectively these findings suggest that breast fibroblast-specific DDR1 mediates collagen deposition and immunomodulatory function within the mammary gland and warrants further investigation as a potential target for fibroblast-modulating therapy in benign and neoplastic breast disorders.
In International Journal of Molecular Sciences on 9 June 2023 by Skandorff, I., Ragonnaud, E., et al.
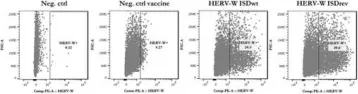
Expression of human endogenous retrovirus type W (HERV-W) has been linked to cancer, making HERV-W antigens potential targets for therapeutic cancer vaccines. In a previous study, we effectively treated established tumours in mice by using adenoviral-vectored vaccines targeting the murine endogenous retrovirus envelope and group-specific antigen (Gag) of melanoma-associated retrovirus (MelARV) in combination with anti-PD-1. To break the immunological tolerance to MelARV, we mutated the immunosuppressive domain (ISD) of the MelARV envelope. However, reports on the immunogenicity of the HERV-W envelope, Syncytin-1, and its ISD are conflicting. To identify the most effective HERV-W cancer vaccine candidate, we evaluated the immunogenicity of vaccines encoding either the wild-type or mutated HERV-W envelope ISD in vitro and in vivo. Here, we show that the wild-type HERV-W vaccine generated higher activation of murine antigen-presenting cells and higher specific T-cell responses than the ISD-mutated counterpart. We also found that the wild-type HERV-W vaccine was sufficient to increase the probability of survival in mice subjected to HERV-W envelope-expressing tumours compared to a control vaccine. These findings provide the foundation for developing a therapeutic cancer vaccine targeting HERV-W-positive cancers in humans.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Int J Mol Sci on 9 June 2023 by Skandorff, I., Ragonnaud, E., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 1