The roles of NK cells in human melanoma remain only partially understood. We characterized NK cells from peripheral blood ex vivo by flow cytometry obtained from late stage (III/IV) melanoma patients. Interestingly, we found that the abundance of CD56bright NK cells negatively correlate with overall patient survival, together with distant metastases, in a multivariate cox regression analysis. The patients' CD56bright NK cells showed upregulation of CD11a, CD38 and CD95 as compared to healthy controls, pointing to an activated phenotype as well as a possible immune regulatory role in melanoma patients. After stimulation in vitro, CD56bright NK cells produced less TNFα and GMCSF in patients than controls. Furthermore, IFNγ production by the CD56bright NK cells correlated inversely with overall survival. Our results highlight that abundance and function of CD56bright NK cells are associated with melanoma patient survival, emphasizing the potential of NK cell subsets for biomarker discovery and future therapeutic targeting.
Product Citations: 6
Circulating CD56bright NK cells inversely correlate with survival of melanoma patients.
In Scientific Reports on 14 March 2019 by de Jonge, K., Ebering, A., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
Prostaglandin E2 suppresses human group 2 innate lymphoid cell function.
In The Journal of Allergy and Clinical Immunology on 1 May 2018 by Maric, J., Ravindran, A., et al.
Group 2 innate lymphoid cells (ILC2s) are involved in the initial phase of type 2 inflammation and can amplify allergic immune responses by orchestrating other type 2 immune cells. Prostaglandin (PG) E2 is a bioactive lipid that plays protective roles in the lung, particularly during allergic inflammation.
We set out to investigate how PGE2 regulates human ILC2 function.
The effects of PGE2 on human ILC2 proliferation and intracellular cytokine and transcription factor expression were assessed by means of flow cytometry. Cytokine production was measured by using ELISA, and real-time quantitative PCR was performed to detect PGE2 receptor expression.
PGE2 inhibited GATA-3 expression, as well as production of the type 2 cytokines IL-5 and IL-13, from human tonsillar and blood ILC2s in response to stimulation with a combination of IL-25, IL-33, thymic stromal lymphopoietin, and IL-2. Furthermore, PGE2 downregulated the expression of IL-2 receptor α (CD25). In line with this observation, PGE2 decreased ILC2 proliferation. These effects were mediated by the combined action of E-type prostanoid receptor (EP) 2 and EP4 receptors, which were specifically expressed on ILC2s.
Our findings reveal that PGE2 limits ILC2 activation and propose that selective EP2 and EP4 receptor agonists might serve as a promising therapeutic approach in treating allergic diseases by suppressing ILC2 function.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
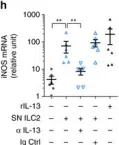
Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis.
In Nature Communications on 19 September 2017 by Trabanelli, S., Chevalier, M. F., et al.
Group 2 innate lymphoid cells (ILC2s) are involved in human diseases, such as allergy, atopic dermatitis and nasal polyposis, but their function in human cancer remains unclear. Here we show that, in acute promyelocytic leukaemia (APL), ILC2s are increased and hyper-activated through the interaction of CRTH2 and NKp30 with elevated tumour-derived PGD2 and B7H6, respectively. ILC2s, in turn, activate monocytic myeloid-derived suppressor cells (M-MDSCs) via IL-13 secretion. Upon treating APL with all-trans retinoic acid and achieving complete remission, the levels of PGD2, NKp30, ILC2s, IL-13 and M-MDSCs are restored. Similarly, disruption of this tumour immunosuppressive axis by specifically blocking PGD2, IL-13 and NKp30 partially restores ILC2 and M-MDSC levels and results in increased survival. Thus, using APL as a model, we uncover a tolerogenic pathway that may represent a relevant immunosuppressive, therapeutic targetable, mechanism operating in various human tumour types, as supported by our observations in prostate cancer.Group 2 innate lymphoid cells (ILC2s) modulate inflammatory and allergic responses, but their function in cancer immunity is still unclear. Here the authors show that, in acute promyelocytic leukaemia, tumour-activated ILC2s secrete IL-13 to induce myeloid-derived suppressor cells and support tumour growth.
-
FC/FACS
-
Cancer Research
Expression and functions of long noncoding RNAs during human T helper cell differentiation.
In Nature Communications on 23 April 2015 by Spurlock, C. F., Tossberg, J. T., et al.
Long noncoding RNAs (lncRNAs) regulate an array of biological processes in cells and organ systems. Less is known about their expression and function in lymphocyte lineages. Here we have identified >2000 lncRNAs expressed in human T-cell cultures and those that display a TH lineage-specific pattern of expression and are intragenic or adjacent to TH lineage-specific genes encoding proteins with immunologic functions. One lncRNA cluster selectively expressed by the effector TH2 lineage consists of four alternatively spliced transcripts that regulate the expression of TH2 cytokines, IL-4, IL-5 and IL-13. Genes encoding this lncRNA cluster in humans overlap the RAD50 gene and thus are contiguous with the previously described TH2 locus control region (LCR) in the mouse. Given its genomic synteny with the TH2-LCR, we refer to this lncRNA cluster as TH2-LCR lncRNA.
-
Genetics
Human Peripheral CD4(+) Vδ1(+) γδT Cells Can Develop into αβT Cells.
In Frontiers in Immunology on 25 February 2015 by Ziegler, H., Welker, C., et al.
The lifelong generation of αβT cells enables us to continuously build immunity against pathogens and malignancies despite the loss of thymic function with age. Homeostatic proliferation of post-thymic naïve and memory T cells and their transition into effector and long-lived memory cells balance the decreasing output of naïve T cells, and recent research suggests that also αβT-cell development independent from the thymus may occur. However, the sites and mechanisms of extrathymic T-cell development are not yet understood in detail. γδT cells represent a small fraction of the overall T-cell pool, and are endowed with tremendous phenotypic and functional plasticity. γδT cells that express the Vδ1 gene segment are a minor population in human peripheral blood but predominate in epithelial (and inflamed) tissues. Here, we characterize a CD4(+) peripheral Vδ1(+) γδT-cell subpopulation that expresses stem-cell and progenitor markers and is able to develop into functional αβT cells ex vivo in a simple culture system and in vivo. The route taken by this process resembles thymic T-cell development. However, it involves the re-organization of the Vδ1(+) γδTCR into the αβTCR as a consequence of TCR-γ chain downregulation and the expression of surface Vδ1(+)Vβ(+) TCR components, which we believe function as surrogate pre-TCR. This transdifferentiation process is readily detectable in vivo in inflamed tissue. Our study provides a conceptual framework for extrathymic T-cell development and opens up a new vista in immunology that requires adaptive immune responses in infection, autoimmunity, and cancer to be reconsidered.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Nat Commun on 19 September 2017 by Trabanelli, S., Chevalier, M. F., et al.
Fig.3.H

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2
In Front Immunol on 25 February 2015 by Ziegler, H., Welker, C., et al.
Fig.3.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2