In autoimmunity, an imbalance of effector (Teff) and regulatory (Treg)T cells contributes to inflammation and tissue destruction. CD2, highly expressed on Teff and at lower levels on Treg and naive T cells (Tn), is an attractive target for depleting Teff at sites of inflammation. SBT115301 is a second generation CD2-targeting fusion protein containing the cognate receptor of CD2, lymphocyte function associated antigen-3 (LFA-3; CD58). In in vitro and in vivo studies, SBT115301 preferentially decreased CD2hi-expressing Teff cells compared to Treg and Tn. In a phase 1 clinical trial, SBT115301 selectively reduced memory T cells. SBT115301 was well tolerated aside from decreases of CD4+ T cells in some participants in the highest dose IM and IV cohorts. Anti-drug antibodies decreased exposure of SBT115301 in some participants without affecting the pharmacodynamics. These data support further study of SBT115301 as a monotherapy or in combination with other drugs in autoimmune indications.
© 2025 Sonoma Biotherapeutics.
Product Citations: 22
In IScience on 16 May 2025 by Lebrec, H., Bui, J., et al.
-
Immunology and Microbiology
CRISPR-Cas9n-mediated ELANE promoter editing for gene therapy of severe congenital neutropenia.
In Molecular Therapy on 5 June 2024 by Nasri, M., Ritter, M. U., et al.
Severe congenital neutropenia (CN) is an inherited pre-leukemia bone marrow failure syndrome commonly caused by autosomal-dominant ELANE mutations (ELANE-CN). ELANE-CN patients are treated with daily injections of recombinant human granulocyte colony-stimulating factor (rhG-CSF). However, some patients do not respond to rhG-CSF, and approximately 15% of ELANE-CN patients develop myelodysplasia or acute myeloid leukemia. Here, we report the development of a curative therapy for ELANE-CN through inhibition of ELANE mRNA expression by introducing two single-strand DNA breaks at the opposing DNA strands of the ELANE promoter TATA box using CRISPR-Cas9D10A nickases-termed MILESTONE. This editing effectively restored defective neutrophil differentiation of ELANE-CN CD34+ hematopoietic stem and progenitor cells (HSPCs) in vitro and in vivo, without affecting the functions of the edited neutrophils. CRISPResso analysis of the edited ELANE-CN CD34+ HSPCs revealed on-target efficiencies of over 90%. Simultaneously, GUIDE-seq, CAST-Seq, and rhAmpSeq indicated a safe off-target profile with no off-target sites or chromosomal translocations. Taken together, ex vivo gene editing of ELANE-CN HSPCs using MILESTONE in the setting of autologous stem cell transplantation could be a universal, safe, and efficient gene therapy approach for ELANE-CN patients.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
In Nature Communications on 18 May 2024 by Zhang, Z., Kean, I. R. L., et al.
Multisystem inflammatory syndrome in children is a post-infectious presentation SARS-CoV-2 associated with expansion of the T cell receptor Vβ21.3+ T-cell subgroup. Here we apply muti-single cell omics to compare the inflammatory process in children with acute respiratory COVID-19 and those presenting with non SARS-CoV-2 infections in children. Here we show that in Multi-Inflammatory Syndrome in Children (MIS-C), the natural killer cell and monocyte population demonstrate heightened CD95 (Fas) and Interleuking 18 receptor expression. Additionally, TCR Vβ21.3+ CD4+ T-cells exhibit skewed differentiation towards T helper 1, 17 and regulatory T cells, with increased expression of the co-stimulation receptors ICOS, CD28 and interleukin 18 receptor. We observe no functional evidence for NLRP3 inflammasome pathway overactivation, though MIS-C monocytes show elevated active caspase 8. This, coupled with raised IL18 mRNA expression in CD16- NK cells on single cell RNA sequencing analysis, suggests interleukin 18 and CD95 signalling may trigger activation of TCR Vβ21.3+ T-cells in MIS-C, driven by increased IL-18 production from activated monocytes and CD16- Natural Killer cells.
© 2024. The Author(s).
-
FC/FACS
-
Immunology and Microbiology
In IScience on 19 May 2023 by Jung, H. S., Suknuntha, K., et al.
Hemogenic endothelium (HE) is the main source of blood cells in the embryo. To improve blood manufacturing from human pluripotent stem cells (hPSCs), it is essential to define the molecular determinants that enhance HE specification and promote development of the desired blood lineage from HE. Here, using SOX18-inducible hPSCs, we revealed that SOX18 forced expression at the mesodermal stage, in contrast to its homolog SOX17, has minimal effects on arterial specification of HE, expression of HOXA genes and lymphoid differentiation. However, forced expression of SOX18 in HE during endothelial-to-hematopoietic transition (EHT) greatly increases NK versus T cell lineage commitment of hematopoietic progenitors (HPs) arising from HE predominantly expanding CD34+CD43+CD235a/CD41a-CD45- multipotent HPs and altering the expression of genes related to T cell and Toll-like receptor signaling. These studies improve our understanding of lymphoid cell specification during EHT and provide a new tool for enhancing NK cell production from hPSCs for immunotherapies.
© 2023 The Author(s).
A topological refactoring design strategy yields highly stable granulopoietic proteins.
In Nature Communications on 26 May 2022 by Skokowa, J., Hernandez Alvarez, B., et al.
Protein therapeutics frequently face major challenges, including complicated production, instability, poor solubility, and aggregation. De novo protein design can readily address these challenges. Here, we demonstrate the utility of a topological refactoring strategy to design novel granulopoietic proteins starting from the granulocyte-colony stimulating factor (G-CSF) structure. We change a protein fold by rearranging the sequence and optimising it towards the new fold. Testing four designs, we obtain two that possess nanomolar activity, the most active of which is highly thermostable and protease-resistant, and matches its designed structure to atomic accuracy. While the designs possess starkly different sequence and structure from the native G-CSF, they show specific activity in differentiating primary human haematopoietic stem cells into mature neutrophils. The designs also show significant and specific activity in vivo. Our topological refactoring approach is largely independent of sequence or structural context, and is therefore applicable to a wide range of protein targets.
© 2022. The Author(s).
-
FC/FACS
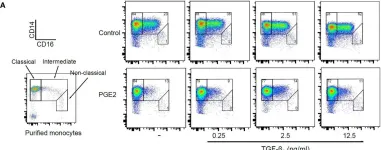
In Front Immunol on 11 July 2018 by Remes Lenicov, F., Paletta, A. L., et al.
Fig.9.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1