Susceptibility to life-threatening influenza increases with age, partly due to declining immunity. Frequency, phenotype and T-cell receptor (TCR) composition of influenza-specific CD8+ T-cells directed at the prominent A2/M158 influenza epitope change across the human lifespan.
We investigated longevity and mechanisms underlying age-related changes in influenza-specific TCR repertoires by performing longitudinal analyses in young and older adults across 7-12 years within A2/M158+CD8+ T-cells using peptide-HLA tetramers directly ex vivo. Paired TCRαβ-chains were used to track clonotypes over time within individuals.
Expanded public and private TCR clonotypes were long-lived but gradually declined over time. Loss of public clonotypes was initially compensated by expansions of clonotypes expressing public-associated features. Once these public-associated TCR clonotypes were abated in older adults, the void was filled by expansions of less similar private TCR clonotypes. Expanded older private TCR clonotypes also declined over time and were gradually replaced by other private TCR clonotypes with low similarity to public TCR clonotypes detected in adults.
Despite our relatively small cohort, we provided conclusive evidence that CD8+ T-cells to a single HLA-A2-restricted influenza-epitope are long-lived. However, dynamic changes occur at the clonotypic level, which eventually result in loss of public clonotypes, indicating that T-cell-based influenza vaccines are likely more effective in adults than older adults.
This research was supported by the National Health and Medical Research Council (#1173871, #1159272), the Australian Research Council (#190102704), European Union's Horizon 2020 (#792532), the University of Melbourne. Funders had no role in design, analysis or reporting of the study.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Product Citations: 83
In EBioMedicine on 1 May 2025 by van de Sandt, C. E., McQuilten, H. A., et al.
-
Immunology and Microbiology
In IScience on 18 April 2025 by Liu, H., Ge, W., et al.
Hepatocellular carcinoma (HCC) resists immunotherapy due to its immunosuppressive microenvironment. Sarcoma homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) inhibits T cell receptor signaling, and its pharmacological inhibition is limited by poor selectivity and membrane permeability. Here, we generated CRISPR-edited SHP-1-knockout (KO) CD8+ T cells to enhance adoptive therapy against HCC. Single-cell RNA sequencing of HCC patient T cells revealed elevated SHP-1 in exhausted subsets. SHP-1-KO T cells exhibited increased effector memory T cells (TEM) proportions and enhanced IFN-γ/Granzyme B/perforin secretion, improving cytotoxicity against HCC lines. In humanized PDX models, SHP-1-KO T cells demonstrated superior tumor-killing activity. Transcriptomics identified upregulated lipid metabolism pathways, with HMGCR as a hub gene. Combining SHP-1-KO T cells with simvastatin (HMGCR inhibitor) synergistically amplified anti-HCC efficacy. This study proposes a dual strategy combining SHP-1-targeted cell therapy and metabolic modulation to overcome immunotherapy resistance, offering a translatable approach for HCC treatment.
© 2025 The Author(s).
-
Cancer Research
In Nature Immunology on 1 March 2025 by Zhu, A., Chen, Z., et al.
Mucosal antigen-specific T cells are pivotal for pathogen clearance and immune modulation in respiratory infections. Dysregulated T cell responses exacerbate coronavirus disease 2019 severity, marked by cytokine storms and respiratory failure. Despite extensive description in peripheral blood, the characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells in the lungs remain elusive. Here we conducted integrated single-cell profiling of SARS-CoV-2-specific T cells in 122 bronchoalveolar lavage fluid (BALF) and 280 blood samples from 159 patients, including 27 paired BALF and blood samples from 24 patients. SARS-CoV-2-specific T cells were robustly elicited in BALF irrespective of prior vaccination, correlating with diminished viral loads, lessened systemic inflammation and improved respiratory function. SARS-CoV-2-specific T cells in BALF exhibited profound activation, along with proliferative and multi-cytokine-producing capabilities and a glycolysis-driven metabolic signature, which were distinct from those observed in peripheral blood mononuclear cells. After viral clearance, these specific T cells maintained a polyfunctional tissue-resident memory phenotype, highlighting their critical roles in infection control and long-term protection.
© 2025. The Author(s).
-
COVID-19
-
Immunology and Microbiology
Phase I study of efineptakin alfa (NT-I7) for the treatment of Kaposi sarcoma.
In Journal for Immunotherapy of Cancer on 6 February 2025 by Ramaswami, R., Kask, A. S., et al.
CD4+ T-cell lymphocytopenia and immune dysfunction are factors that drive the onset and persistence of Kaposi sarcoma (KS) in people with (PWH) and without HIV. Standard chemotherapy agents for KS can contribute to increasing CD4+ T cell lymphocytopenia. IL-7 is a cytokine that is essential in T-cell development, proliferation and homeostasis. In PWH, IL-7 administration leads to increased numbers of circulating central memory and naïve T-cell phenotypes.
In this multicenter phase I study with a 3+3 dose escalation design, participants with KS with or without HIV received up to four intramuscular injections of IL-7 (NT-I7) every 9 weeks. The primary endpoint of the study was to evaluate safety over three escalating dose levels (DL) of NT-I7 (DL1:480 µg/kg, DL2: 960 µg/kg and DL3: 1200 µg/kg) and identify a maximum tolerated dose. Secondary endpoints included evaluation of antitumor activity per the modified AIDS Clinical Trials Group Criteria and assessment of the effect of NT-I7 on the kinetics of CD4+ and CD8+ T-cells.
Eight cisgender male participants (five with HIV infection) were enrolled. Six participants were treated at DL1, and two were treated at DL2. The study was closed to accrual after enrolment of the second participant on DL2 due to termination of study funding. Four of the eight participants (three in DL1 and one in DL2) completed all four doses of the NT-I7. With regard to treatment-emergent adverse events (AEs), all participants had
-
Cancer Research
In Nature Immunology on 1 February 2025 by Song, F., Tsahouridis, O., et al.
Chimeric antigen receptor T cells (CAR T cells) with T stem (TSCM) cell-like phenotypic characteristics promote sustained antitumor effects. We performed an unbiased and automated high-throughput screen of a kinase-focused compound set to identify kinase inhibitors (KIs) that preserve human TSCM cell-like CAR T cells. We identified three KIs, UNC10225387B, UNC10225263A and UNC10112761A, that combined in vitro increased the frequency of CD45RA+CCR7+TCF1hi TSCM cell-like CAR T cells from both healthy donors and patients with cancer. KI-treated CAR T cells showed enhanced antitumor effects both in vitro and in vivo in mouse tumor models. The KI cocktail maintains TSCM cell-like phenotype preferentially in CAR T cells originating from naive T cells and causes transcriptomic changes without arresting T cell activation or modulating the chromatin organization. Specific kinases, ITK, ADCK3, MAP3K4 and CDK13, targeted by the KI cocktail in a dose-dependent manner are directly associated with the preservation of TSCM cell-like CAR T cells. Knockdown of these kinases individually or in combination enriches for TSCM cell-like CAR T cells, but only CAR T cells generated in the presence of the KI cocktail show robust expansion and differentiation on stimulation with tumor cells. Overall, transient pharmacological inhibition of strategically targeted kinases maintains stem-like features in CAR T cells and improves their antitumor activity.
© 2025. The Author(s).
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Elife on 21 September 2023 by Herzfeldt, A. K., Gamez, M. P., et al.
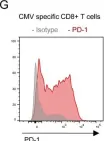
Fig.4.G

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 3
In Oncol Lett on 1 October 2014 by Han, L., Liu, F., et al.
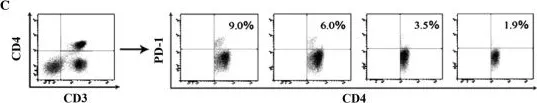
Fig.3.C

-
MACS
-
Homo sapiens (Human)
Collected and cropped from Oncol Lett by CiteAb, provided under a CC-BY license
Image 1 of 3
In Oncol Lett on 1 October 2014 by Han, L., Liu, F., et al.
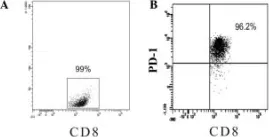
Fig.4.A

-
MACS
-
Homo sapiens (Human)
Collected and cropped from Oncol Lett by CiteAb, provided under a CC-BY license
Image 1 of 3