Despite causative links to lymphoproliferative disorders, little is known about early events governing KSHV infection in B lymphocytes. IL-6 signaling plays a critical role in KSHV-mediated disease, with human IL-6 (hIL6) levels correlating with viral load and disease progression. This dynamic is even more complex due to the coexistence of hIL6 and KSHV-encoded viral IL-6 (vIL6) in these diseases. We hypothesize that hIL6 and vIL6 play critical, separable and collective roles in the early stages of KSHV infection in B cells. In this study, we use our ex vivo model of KSHV infection in human tonsil lymphocytes to investigate the relative contributions of vIL6 and hIL6 to early infection events in human B cells. We demonstrate that vIL6 and hIL6 collectively suppress KSHV infection in B cells restricting the distribution of KSHV within B cell subsets. We show that vIL6 manipulates hIL6 expression in a subset-specific manner, and that vIL6 and hIL6 differentially influence the differentiation of germinal center and plasmablasts. Taken together, these results suggest a novel paradigm in which KSHV uses vIL6 to abrogate the GC-mediated maturation pathway for antibody secreting cells that is driven by hIL6 signaling.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Product Citations: 33
KSHV vIL6 Inhibits Functional B Cell Maturation During De Novo Infection.
In Journal of Medical Virology on 1 July 2025 by Zakir, W., Osborn, J. M., et al.
-
Immunology and Microbiology
Interferon activation in bone marrow long-lived plasma cells in systemic lupus erythematosus.
In Frontiers in Immunology on 27 January 2025 by Alzamareh, D. F., Meednu, N., et al.
While durable antibody responses from long-lived plasma cell (LLPC) populations are important for protection against pathogens, LLPC may be harmful if they produce antibodies against self-proteins or self-nuclear antigens as occurs in autoimmune diseases such as systemic lupus erythematosus (SLE). Thus, the elimination of autoreactive LLPC may improve the treatment of antibody-driven autoimmune diseases. However, LLPC remain a challenging therapeutic target. Here, we compare the matched bone marrow (BM) and peripheral blood (PBL) plasma cell (PC) compartments of SLE and healthy donors (HD). We show a similar distribution of CD138- and CD138+ PC, including putative LLPC (CD19- CD138+ CD38+), between SLE and HD BM. For both SLE and HD, CD138+ PC are at a higher frequency in BM than PBL. Expression of Ki-67 associates with the PBL compartment where it is found on all PC subsets regardless of CD19 or CD138 expression. Transcriptomic analysis identifies an interferon (IFN) gene signature in transitional B cells in the SLE BM, but surprisingly also in the BM PC derived from SLE. BM PC and B cells phosphorylate STAT1 in response to type I IFN stimulation in vitro, but with decreased fold change compared to those from the PBL. While BM PC bind type I IFN receptor-blocking antibody anifrolumab, it is to a lesser degree than circulating B cells. Anti-nuclear autoantibodies (ANA) are found in the BM supernatant and PBL serum of SLE patients. Both SLE and HD BM-derived PC have increased survival compared to their PBL counterparts when treated with verdinexor. In summary, these findings show evidence of IFN activation in BM PC from SLE.
Copyright © 2025 Alzamareh, Meednu, Nandedkar-Kulkarni, Krenitsky, Barnard, Yasaka, Durrett, Thakar, Rangel-Moreno, Anolik and Barnas.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Nature Immunology on 1 January 2025 by Faliti, C. E., Van, T. T. P., et al.
Severe acute respiratory syndrome coronavirus 2 mRNA vaccination has reduced effectiveness in certain immunocompromised individuals. However, the cellular mechanisms underlying these defects, as well as the contribution of disease-induced cellular abnormalities, remain largely unexplored. In this study, we conducted a comprehensive serological and cellular analysis of patients with autoimmune systemic lupus erythematosus (SLE) who received the Wuhan-Hu-1 monovalent mRNA coronavirus disease 2019 vaccine. Our findings revealed that patients with SLE exhibited reduced avidity of anti-receptor-binding domain antibodies, leading to decreased neutralization potency and breadth. We also observed a sustained anti-spike response in IgD-CD27- 'double-negative (DN)' DN2/DN3 B cell populations persisting during memory responses and with greater representation in the SLE cohort. Additionally, patients with SLE displayed compromised anti-spike T cell immunity. Notably, low vaccine efficacy strongly correlated with higher values of a newly developed extrafollicular B and T cell score, supporting the importance of distinct B cell endotypes. Finally, we found that anti-BAFF blockade through belimumab treatment was associated with poor vaccine immunogenicity due to inhibition of naive B cell priming and an unexpected impact on circulating T follicular helper cells.
© 2024. The Author(s).
-
COVID-19
-
Genetics
-
Immunology and Microbiology
In Nature Communications on 16 September 2024 by van der Lans, S. P. A., Bardoel, B. W., et al.
Antibody-dependent complement activation plays a key role in the natural human immune response to infections. Currently, the understanding of which antibody-antigen combinations drive a potent complement response on bacteria is limited. Here, we develop an antigen-agnostic approach to stain and single-cell sort human IgG memory B cells recognizing intact bacterial cells, keeping surface antigens in their natural context. With this method we successfully identified 29 antibodies against K. pneumoniae, a dominant cause of hospital-acquired infections with increasing antibiotic resistance. Combining genetic tools and functional analyses, we reveal that the capacity of antibodies to activate complement on K. pneumoniae critically depends on their antigenic target. Furthermore, we find that antibody combinations can synergistically activate complement on K. pneumoniae by strengthening each other's binding in an Fc-independent manner. Understanding the molecular basis of effective complement activation by antibody combinations to mimic a polyclonal response could accelerate the development of antibody-based therapies against problematic infections.
© 2024. The Author(s).
-
FC/FACS
-
Immunology and Microbiology
In Cell Reports on 23 July 2024 by Pelletier, A. N., Sánchez, G. P., et al.
Protective immunity to dengue virus (DENV) requires antibody response to all four serotypes. Systems vaccinology identifies a multi-OMICs pre-vaccination signature and mechanisms predictive of broad antibody responses after immunization with a tetravalent live attenuated DENV vaccine candidate (Butantan-DV/TV003). Anti-inflammatory pathways, including TGF-β signaling expressed by CD68low monocytes, and the metabolites phosphatidylcholine (PC) and phosphatidylethanolamine (PE) positively correlate with broadly neutralizing antibody responses against DENV. In contrast, expression of pro-inflammatory pathways and cytokines (IFN and IL-1) in CD68hi monocytes and primary and secondary bile acids negatively correlates with broad DENV-specific antibody responses. Induction of TGF-β and IFNs is done respectively by PC/PE and bile acids in CD68low and CD68hi monocytes. The inhibition of viral sensing by PC/PE-induced TGF-β is confirmed in vitro. Our studies show that the balance between metabolites and the pro- or anti-inflammatory state of innate immune cells drives broad and protective B cell response to a live attenuated dengue vaccine.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
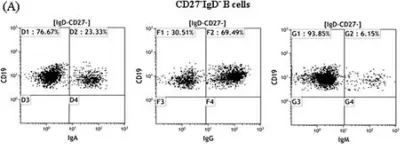
In Arthritis Res Ther on 14 March 2015 by Mahmood, Z., Muhammad, K., et al.
Fig.2.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Arthritis Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 1