APOE4 is the strongest genetic risk factor for Alzheimer's disease (AD), with increased odds ratios in female carriers. Targeting amyloid plaques shows modest improvement in male non-APOE4 carriers. Leveraging single-cell transcriptomics across APOE variants in both sexes, multiplex flow cytometry and validation in two independent cohorts of APOE4 female carriers with AD, we identify a new subset of neutrophils interacting with microglia associated with cognitive impairment. This phenotype is defined by increased interleukin (IL)-17 and IL-1 coexpressed gene modules in blood neutrophils and in microglia of cognitively impaired female APOE ε4 carriers, showing increased infiltration to the AD brain. APOE4 female IL-17+ neutrophils upregulated the immunosuppressive cytokines IL-10 and TGFβ and immune checkpoints, including LAG3 and PD-1, associated with accelerated immune aging. Deletion of APOE4 in neutrophils reduced this immunosuppressive phenotype and restored the microglial response to neurodegeneration, limiting plaque pathology in AD mice. Mechanistically, IL-17F upregulated in APOE4 neutrophils interacts with microglial IL-17RA to suppress the induction of the neurodegenerative phenotype, and blocking this axis supported cognitive improvement in AD mice. These findings provide a translational basis to target IL-17F in APOE ε4 female carriers with cognitive impairment.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Product Citations: 47
In Nature Medicine on 1 October 2024 by Rosenzweig, N., Kleemann, K. L., et al.
-
Neuroscience
Protocol to construct humanized mice with adult CD34+ hematopoietic stem and progenitor cells.
In STAR Protocols on 20 September 2024 by Yu, C. I., Maser, R., et al.
Humanized mice, defined as mice with human immune systems, have become an emerging model to study human hematopoiesis, infectious disease, and cancer. Here, we describe the techniques to generate humanized NSGF6 mice using adult human CD34+ hematopoietic stem and progenitor cells (HSPCs). We describe steps for constructing and monitoring the engraftment of humanized mice. We then detail procedures for tissue processing and immunophenotyping by flow cytometry to evaluate the multilineage hematopoietic differentiation. For complete details on the use and execution of this protocol, please refer to Yu et al.1.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Engraftment of adult hematopoietic stem and progenitor cells in a novel model of humanized mice.
In IScience on 15 March 2024 by Yu, C. I., Maser, R., et al.
Pre-clinical use of humanized mice transplanted with CD34+ hematopoietic stem and progenitor cells (HSPCs) is limited by insufficient engraftment with adult non-mobilized HSPCs. Here, we developed a novel immunodeficient mice based on NOD-SCID-Il2γc-/- (NSG) mice to support long-term engraftment with human adult HSPCs. As both Flt3L and IL-6 are critical for many aspects of hematopoiesis, we knock-out mouse Flt3 and knock-in human IL6 gene. The resulting mice showed an increase in the availability of mouse Flt3L to human cells and a dose-dependent production of human IL-6 upon activation. Upon transplantation with low number of human HSPCs from adult bone marrow, these humanized mice demonstrated a significantly higher engraftment with multilineage differentiation of human lymphoid and myeloid cells, and tissue colonization at one year after adult HSPC transplant. Thus, these mice enable studies of human hematopoiesis and tissue colonization over time and may facilitate building autologous models for immuno-oncology studies.
© 2024 The Author(s).
In IScience on 15 December 2023 by Liu, S., Liu, Z. C., et al.
Intercellular adhesion molecule 1 (ICAM-1) plays prominent roles in mediating cell-cell adhesion which also facilitates B cell activation and differentiation with the help from CD4+ T cells. Here, we have reported a unique phenomenon that increased ICAM-1 on purified human CD4+ T cells upon anti-CD3/CD28 stimulation enhanced CD4+ T-B cell adhesion whereas induced less B cell differentiation and IgG production. This was largely due to increased PD-1 expression on CD19hi B cells after coculturing with hyperactivated CD4+ T cells. Consequently, ICAM-1 blockade during CD4+ T cell-B cell coculture promoted IgG production with the activation of ERK1/2 and Blimp-1/IRF4 upregulation. Consistently, CD4+ T cells from moderate-to-severe SLE patients with high ICAM-1 expression mediated less IgG production after T-B coculture. Therefore, ICAM-1-mediated human CD4+ T-B cell adhesion provides dual roles on B cell differentiation and IgG production partially depending on expression levels of PD-1 on B cells, supporting cell adhesion and subsequent PD-1 induction as an alternative intrinsic checkpoint for B cell differentiation.
© 2023 The Authors.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Cell Host & Microbe on 11 October 2023 by Banga, R., Procopio, F. A., et al.
Although gut and lymph node (LN) memory CD4 T cells represent major HIV and simian immunodeficiency virus (SIV) tissue reservoirs, the study of the role of dendritic cells (DCs) in HIV persistence has long been limited to the blood due to difficulties to access lymphoid tissue samples. In this study, we show that LN migratory and resident DC subpopulations harbor distinct phenotypic and transcriptomic profiles. Interestingly, both LN DC subpopulations contain HIV intact provirus and inducible replication-competent HIV despite the expression of the antiviral restriction factor SAMHD1. Notably, LN DC subpopulations isolated from HIV-infected individuals treated for up to 14 years are transcriptionally silent but harbor replication-competent virus that can be induced upon TLR7/8 stimulation. Taken together, these results uncover a potential important contribution of LN DCs to HIV infection in the presence of ART.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
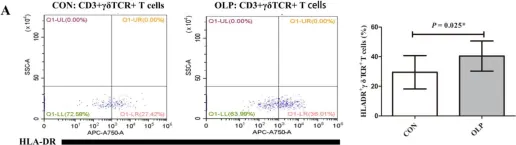
In Biomedicines on 20 March 2023 by Huang, S., Tan, Y. Q., et al.
Fig.3.A

-
FC/FACS
-
Collected and cropped from Biomedicines by CiteAb, provided under a CC-BY license
Image 1 of 1