The aim of this study was to investigate the regenerative effect of lyophilized dental follicle mesenchymal stem cells (DF-MSCs) combined with rat platelet-rich fibrin (PRF) on geriatric skin wounds. Human DF-MSCs which were isolated from the wisdom teeth of healthy donors and PRF were mixed and incubated in a 37 °C incubator for 1-2 h containing 1 million cells in 150 mg PRF. The mixture was suspended in a freeze-drying solution and then lyophilized. Wounds were created on the back skin of Wistar albino rats using a 6 mm punch. Lyophilized DF-MSCs, PRF, or PRF + DF-MSCs were applied to the wounds of rats. On the 15th day, the wound area was histopathologically evaluated in rats. Blood samples from rats were analyzed for total antioxidant status (TAOS), and inflammatory cytokine levels using ELISA. In both young and geriatric rats treated with lyophilized PRF + DF-MSCs, wound area began to significantly decrease from the 10th day compared to the untreated group (p < 0.05). Histopathological examination revealed that in the lyophilized PRF + DF-MSCs treated groups, epithelial integrity and scarless healing significantly increased compared to the untreated groups (p < 0.05). There were no significant differences in TAOS, total oxidant status (TOS), tumor necrosis factor (TNF), interleukin-6 (IL6), and hydroxyproline levels in serum samples from young rats on the 15th day. In geriatric rats, hydroxyproline (HYPS) levels were increased in the DF-MSC and PRF + DF-MSC groups (p < 0.01), TNF was significantly elevated in PRF geriatric group and IL6 was increased in the PRF group compared to the control group (p = 0.01). Lyophilized PRF + DF-MSCs, which is a shelf-stable and ready-to-use product, hold promise, especially for traumatic wounds in geriatric individuals with longer healing times.
© 2025. The Author(s).
Product Citations: 20
In Scientific Reports on 24 February 2025 by Bulut, O., Genç, D., et al.
-
Stem Cells and Developmental Biology
In Clinical Cancer Research on 1 November 2024 by Freeland, J., Muñoz, M., et al.
High-grade complex karyotype sarcomas are a heterogeneous group of tumors with a uniformly poor prognosis. Within complex karyotype sarcomas, there are innumerable genetic changes but identifying those that are clinically relevant has been challenging.
To address this, we utilized a pooled genetic screening approach, informed by The Cancer Genome Atlas (TCGA) data, to identify key drivers and modifiers of sarcoma development that were validated in vivo.
YAP1 and wild-type KRAS were validated as drivers and transformed human mesenchymal stem cells into two distinct sarcoma subtypes, undifferentiated pleomorphic sarcoma and myxofibrosarcoma, respectively. A subset of tumors driven by CDK4 and PIK3CA reflected leiomyosarcoma and osteosarcoma demonstrating the plasticity of this approach and the potential to investigate sarcoma subtype heterogeneity. All generated tumors histologically reflected human sarcomas and had increased aneuploidy as compared to simple karyotype sarcomas. Comparing differential gene expression of TCGA samples to model data identified increased oxidative phosphorylation signaling in YAP1 tumors. Treatment of a panel of soft tissue sarcomas with a combination of YAP1 and oxidative phosphorylation inhibitors led to significantly decreased viability.
Transcriptional co-analysis of TCGA patient samples to YAP1 and KRAS model tumors supports that these sarcoma subtypes lie along a spectrum of disease and adds guidance for further transcriptome-based refinement of sarcoma subtyping. This approach can be used to begin to understand pathways and mechanisms driving human sarcoma development, the relationship between sarcoma subtypes, and to identify and validate new therapeutic vulnerabilities for this aggressive and heterogeneous disease.
©2024 American Association for Cancer Research.
-
Cancer Research
-
Genetics
In Cell Reports on 25 July 2023 by Park, C. S., Yoshihara, H., et al.
The bone marrow microenvironment (BME) drives drug resistance in acute lymphoblastic leukemia (ALL) through leukemic cell interactions with bone marrow (BM) niches, but the underlying mechanisms remain unclear. Here, we show that the interaction between ALL and mesenchymal stem cells (MSCs) through integrin β1 induces an epithelial-mesenchymal transition (EMT)-like program in MSC-adherent ALL cells, resulting in drug resistance and enhanced survival. Moreover, single-cell RNA sequencing analysis of ALL-MSC co-culture identifies a hybrid cluster of MSC-adherent ALL cells expressing both B-ALL and MSC signature genes, orchestrated by a WNT/β-catenin-mediated EMT-like program. Blockade of interaction between β-catenin and CREB binding protein impairs the survival and drug resistance of MSC-adherent ALL cells in vitro and results in a reduction in leukemic burden in vivo. Targeting of this WNT/β-catenin-mediated EMT-like program is a potential therapeutic approach to overcome cell extrinsically acquired drug resistance in ALL.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Cancer Research
In Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society on 1 January 2023 by Margolis, D. J., Mitra, N., et al.
The goal of this multicentre study was to evaluate whether circulating endothelial precursor cells and microparticles can predict diabetic foot ulcer healing by the 16th week of care. We enrolled 207 subjects, and 40.0% (28.4, 41.5) healed by the 16th week of care. Using flow cytometry analysis, several circulating endothelial precursor cells measured at the first week of care were associated with healing after adjustment for wound area and wound duration. For example, CD34+ CD45dim , the univariate odds ratio was 1.19 (95% confidence interval: 0.88, 1.61) and after adjustment for wound area and wound duration, the odds ratio was (1.67 (1.16, 2.42) p = 0.006). A prognostic model using CD34+ CD45dim , wound area, and wound duration had an area under the curve of 0.75 (0.67, 0.82) and CD34+ CD45dim per initial wound area, an area under the curve of 0.72 (0.64, 0.79). Microparticles were not associated with a healed wound. Previous studies have indicated that circulating endothelial precursor cells measured at the first office visit are associated with a healed diabetic foot ulcer. In this multicentred prospective study, we confirm this finding, show the importance of adjusting circulating endothelial precursor cells measurements by wound area, and show circulating endothelial precursor cells per wound area is highly predictive of a healed diabetic foot ulcer by 16th week of care.
© 2022 The Wound Healing Society.
In Frontiers in Cell and Developmental Biology on 8 November 2022 by Mathur, N., Severinsen, M. C. K., et al.
Abdominal obesity associates with cardiometabolic disease and an accumulation of lipids in the visceral adipose depot, whereas lipid accumulation in the subcutaneous depot is more benign. We aimed to further investigate whether the adipogenic properties where cell-intrinsic, or dependent on a depot-specific or obesity-produced microenvironment. We obtained visceral and subcutaneous biopsies from non-obese women (n = 14) or women living with morbid obesity (n = 14) and isolated adipose stem and progenitor cells (ASPCs) from the stromal vascular fraction of non-obese (n = 13) and obese (n = 13). Following in vitro differentiation into mature adipocytes, we observed a contrasting pattern with a lower gene expression of adipogenic markers and a higher gene expression of immunogenic markers in the visceral compared to the subcutaneous adipocytes. We identified the immunogenic factor BST2 as a marker for visceral ASPCs. The effect of obesity and insulin resistance on adipogenic and immunogenic markers in the in vitro differentiated cells was minor. In contrast, differentiation with exogenous Tumor necrosis factor resulted in increased immunogenic signatures, including increased expression of BST2, and decreased adipogenic signatures in cells from both depots. Our data, from 26 women, underscore the intrinsic differences between human visceral and subcutaneous adipose stem and progenitor cells, suggest that dysregulation of adipocytes in obesity mainly occurs at a post-progenitor stage, and highlight an inflammatory microenvironment as a major constraint of human adipogenesis.
Copyright © 2022 Mathur, Severinsen, Jensen, Naver, Schrölkamp, Laye, Watt, Nielsen, Krogh-Madsen, Pedersen and Scheele.
-
FC/FACS
-
Homo sapiens (Human)
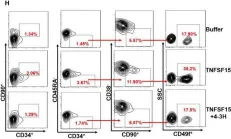
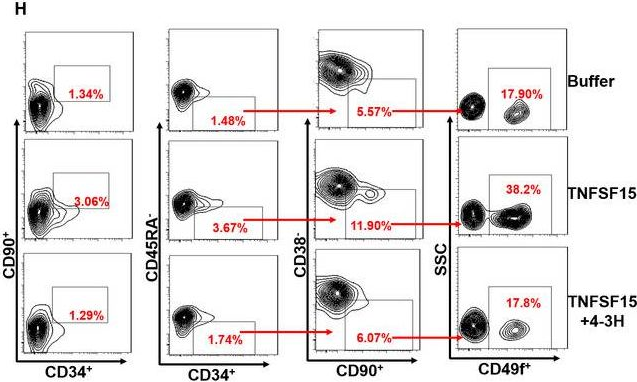
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.1.H
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2
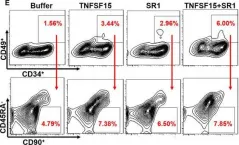
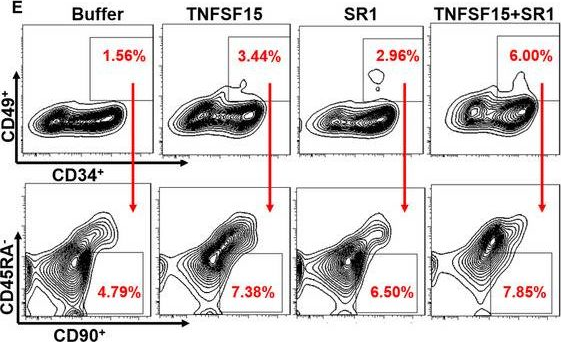
In J Cell Mol Med on 1 October 2020 by Ding, Y., Gao, S., et al.
Fig.3.E
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2