Fungal pathogens often undergo morphological switches, including cell size changes, to adapt to the host environment and cause disease. The pathogenic yeast Cryptococcus neoformans forms so-called 'titan cells' during infection. Titan cells are large, polyploid, display alterations in cell wall and capsule, and are more resistant to phagocytosis and various types of stress. Titan cell formation is regulated by the cAMP/PKA signal pathway, which is stimulated by the protein Gpa1. Here, we show that Gpa1 is activated through phosphorylation by a CDK-related kinase (Crk1), which is targeted for degradation by an E3 ubiquitin ligase (Fbp1). Strains overexpressing CRK1 or an allele lacking a PEST domain exhibit increased production of titan cells similarly to the fbp1∆ mutant. Conversely, CRK1 deletion results in reduced titan cell production, indicating that Crk1 stimulates titan cell formation. Crk1 phosphorylates Gpa1, which then localizes to the plasma membrane and activates the cAMP/PKA signal pathway to induce cell enlargement. Furthermore, titan cell-overproducing strains trigger increased Th1 and Th17 cytokine production in CD4+ T cells and show attenuated virulence in a mouse model of systemic cryptococcosis. Overall, our study provides insights into the regulation of titan cell formation and fungal virulence.
© 2022. The Author(s).
Product Citations: 10
In Nature Communications on 27 October 2022 by Cao, C., Wang, K., et al.
-
FC/FACS
-
Mus musculus (House mouse)
Tumor-Promoting Ly-6G+ SiglecFhigh Cells Are Mature and Long-Lived Neutrophils.
In Cell Reports on 22 September 2020 by Pfirschke, C., Engblom, C., et al.
Myeloid cells co-expressing the markers CD11b, Ly-6G, and SiglecF can be found in large numbers in murine lung adenocarcinomas and accelerate cancer growth by fostering tumor cell invasion, angiogenesis, and immunosuppression; however, some of these cells' fundamental features remain unexplored. Here, we show that tumor-infiltrating CD11b+ Ly-6G+ SiglecFhigh cells are bona fide mature neutrophils and therefore differ from other myeloid cells, including SiglecFhigh eosinophils, SiglecFhigh macrophages, and CD11b+ Ly-6G+ myeloid-derived suppressor cells. We further show that SiglecFhigh neutrophils gradually accumulate in growing tumors, where they can live for several days; this lifespan is in marked contrast to that of their SiglecFlow counterparts and neutrophils in general, which live for several hours only. Together, these findings reveal distinct attributes for tumor-promoting SiglecFhigh neutrophils and help explain their deleterious accumulation in the tumor bed.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Cancer Research
A bioenergetic shift is required for spermatogonial differentiation.
In Cell Discovery on 31 August 2020 by Chen, W., Zhang, Z., et al.
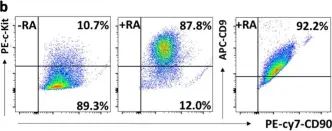
A bioenergetic balance between glycolysis and mitochondrial respiration is particularly important for stem cell fate specification. It however remains to be determined whether undifferentiated spermatogonia switch their preference for bioenergy production during differentiation. In this study, we found that ATP generation in spermatogonia was gradually increased upon retinoic acid (RA)-induced differentiation. To accommodate this elevated energy demand, RA signaling concomitantly switched ATP production in spermatogonia from glycolysis to mitochondrial respiration, accompanied by increased levels of reactive oxygen species. Disrupting mitochondrial respiration significantly blocked spermatogonial differentiation. Inhibition of glucose conversion to glucose-6-phosphate or pentose phosphate pathway also repressed the formation of c-Kit+ differentiating germ cells, suggesting that metabolites produced from glycolysis are required for spermatogonial differentiation. We further demonstrated that the expression levels of several metabolic regulators and enzymes were significantly altered upon RA-induced differentiation, with both RNA-seq and quantitative proteomic analyses. Taken together, our data unveil a critically regulated bioenergetic balance between glycolysis and mitochondrial respiration that is required for spermatogonial proliferation and differentiation.
© The Author(s) 2020.
-
FC/FACS
-
Mus musculus (House mouse)
The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells.
In Immunity on 16 July 2019 by Omenetti, S., Bussi, C., et al.
T helper 17 (Th17) cells are pathogenic in many inflammatory diseases, but also support the integrity of the intestinal barrier in a non-inflammatory manner. It is unclear what distinguishes inflammatory Th17 cells elicited by pathogens and tissue-resident homeostatic Th17 cells elicited by commensals. Here, we compared the characteristics of Th17 cells differentiating in response to commensal bacteria (SFB) to those differentiating in response to a pathogen (Citrobacter rodentium). Homeostatic Th17 cells exhibited little plasticity towards expression of inflammatory cytokines, were characterized by a metabolism typical of quiescent or memory T cells, and did not participate in inflammatory processes. In contrast, infection-induced Th17 cells showed extensive plasticity towards pro-inflammatory cytokines, disseminated widely into the periphery, and engaged aerobic glycolysis in addition to oxidative phosphorylation typical for inflammatory effector cells. These findings will help ensure that future therapies directed against inflammatory Th17 cells do not inadvertently damage the resident gut population.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
PD-1 Is Involved in the Dysregulation of Type 2 Innate Lymphoid Cells in a Murine Model of Obesity.
In Cell Reports on 20 November 2018 by Oldenhove, G., Boucquey, E., et al.
Recent observations clearly highlight the critical role of type 2 innate lymphoid cells in maintaining the homeostasis of adipose tissues in humans and mice. This cell population promotes beiging and limits adiposity directly and indirectly by sustaining a Th2-prone environment enriched in eosinophils and alternatively activated macrophages. Accordingly, the number and function of type 2 innate lymphoid cells (ILC2s) are strongly impaired in obese individuals. In this work, we identify the PD-1-PD-L1 pathway as a factor leading to ILC2 destabilization upon high-fat feeding resulting in impaired tissue metabolism. Tumor necrosis factor (TNF) appears to play a central role, triggering interleukin-33 (IL-33)-dependent PD-1 expression on ILC2s and recruiting and activating PD-L1hi M1 macrophages. PD-1 blockade partially restores the type 2 innate axis, raising the possibility of restoring tissue homeostasis.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
In Cell Discov on 31 August 2020 by Chen, W., Zhang, Z., et al.
Fig.1.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cell Discov by CiteAb, provided under a CC-BY license
Image 1 of 1