Tissue engineering is a promising approach for the production of small-diameter vascular grafts; however, there are limited data directly comparing the suitability of applicable cell types for vessel biofabrication. Here, we investigated the potential of adult smooth muscle cells (SMCs), placental mesenchymal stem cells (MSCs), placental endothelial colony-forming cells (ECFCs), and a combination of MSCs and ECFCs on highly porous biocompatible poly(ɛ-caprolactone) (PCL) scaffolds produced via melt electrowriting (MEW) for the biofabrication of tissue-engineered vascular grafts (TEVGs). Cellular attachment, proliferation, and deposition of essential extracellular matrix (ECM) components were analysed in vitro over four weeks. TEVGs cultured with MSCs accumulated the highest levels of collagenous components within a dense ECM, while SMCs and the coculture were more sparsely populated, ascertained via histological and immunofluorescence imaging, and biochemical assessment. Scanning electron microscopy (SEM) enabled visualisation of morphological differences in cell attachment and growth, with MSCs and SMCs infiltrating and covering scaffolds completely within the 28-day culture period. Coverage and matrix deposition by ECFCs was limited. However, ECFCs lined the ECM formed by MSCs in coculture, visualised via immunostaining. Thus, of cells investigated, placental MSCs were identified as the preferred cell source for the fabrication of tissue-engineered constructs, exhibiting extensive population of porous polymer scaffolds and production of ECM components; with the inclusion of ECFCs for luminal endothelialisation, an encouraging outcome warranting further consideration in future studies. In combination, these findings represent a substantial step toward the development of the next generation of small-diameter vascular grafts in the management of cardiovascular disease.
Copyright © 2024 Angus Weekes et al.
Product Citations: 17
In Journal of Tissue Engineering and Regenerative Medicine on 14 April 2025 by Weekes, A., Wasielewska, J. M., et al.
-
Stem Cells and Developmental Biology
In Physiological Reports on 1 February 2025 by Landers-Ramos, R. Q., Kim, K., et al.
Peripheral blood mononuclear cells (PBMCs) represent a heterogeneous mix of cells with paracrine functions that may be altered following prolonged exercise. We determined the effect of ultramarathon running on PBMC paracrine function and PBMC subtype number. Recreational athletes participated in a 50 km ultramarathon. Blood was sampled from N = 7 at baseline, 10 km, 50 km, and 24 h post-race. PBMCs were isolated and cultured, and conditioned media was used for a HUVEC-based proliferation assay. CD31+, CD3+, and CD31+/CD3+ PBMCs were quantified at each time point. Proliferation increased from baseline to 50 km (p = 0.004) and was reduced from 50 km to 24 h post (p = 0.008). There was an increase in CD31+ PBMCs after 50 km (p = 0.014), returning to baseline at 24 h post-race (p = 0.246). CD3+ PBMC and CD31+/CD3+ PBMC numbers were reduced after 50 km (p = 0.001 and p = 0.002, respectively), returning to baseline levels 24 h post-race (p = 0.190 and p = 0.315, respectively). PBMC paracrine activity following a 50 km enhances endothelial cell proliferation. Alterations in PBMC subtypes after 50 km suggest a protective role of PBMCs in response to prolonged stresses of ultramarathon running.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
-
Cardiovascular biology
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Cells on 26 August 2024 by Kempf, S., Popp, R., et al.
The pericyte coverage of microvessels is altered in metabolic diseases, but the mechanisms regulating pericyte-endothelial cell communication remain unclear. This study investigated the formation and function of pericyte tunneling nanotubes (TNTs) and their impact on endothelial cell metabolism. TNTs were analyzed in vitro in retinas and co-cultures of pericytes and endothelial cells. Using mass spectrometry, the influence of pericytes on endothelial cell metabolism was examined. TNTs were present in the murine retina, and although diabetes was associated with a decrease in pericyte coverage, TNTs were longer. In vitro, pericytes formed TNTs in the presence of PDGF, extending toward endothelial cells and facilitating mitochondrial transport from pericytes to endothelial cells. In experiments with mitochondria-depleted endothelial cells displaying defective TCA cycle metabolism, pericytes restored the mitochondrial network and metabolism. 19,20-Dihydroxydocosapentaenoic acid (19,20-DHDP), known to disrupt pericyte-endothelial cell junctions, prevented TNT formation and metabolic rescue in mitochondria-depleted endothelial cells. 19,20-DHDP also caused significant changes in the protein composition of pericyte-endothelial cell junctions and involved pathways related to phosphatidylinositol 3-kinase, PDGF receptor, and RhoA signaling. Pericyte TNTs contact endothelial cells and support mitochondrial transfer, influencing metabolism. This protective mechanism is disrupted by 19,20-DHDP, a fatty acid mediator linked to diabetic retinopathy.
-
Cell Biology
Preprint on BioRxiv : the Preprint Server for Biology on 12 February 2024 by Ru, Y., Ma, M., et al.
Osteogenic differentiation is essential for bone development and metabolism, but the underlying gene regulatory networks have not been well investigated. We differentiated mesenchymal stem cells, derived from 20 human induced pluripotent stem cell lines, into preosteoblasts and osteoblasts, and performed systematic RNA-seq analyses of 60 samples for differential gene expression. We noted a highly significant correlation in expression patterns and genomic proximity among transcription factor (TF) and long noncoding RNA (lncRNA) genes. We identified TF-TF regulatory networks, regulatory roles of lncRNAs on their neighboring coding genes for TFs and splicing factors, and differential splicing of TF, lncRNA, and splicing factor genes. TF-TF regulatory and gene co-expression network analyses suggested an inhibitory role of TF KLF16 in osteogenic differentiation. We demonstrate that in vitro overexpression of human KLF16 inhibits osteogenic differentiation and mineralization, and in vivo Klf16 +/- mice exhibit increased bone mineral density, trabecular number, and cortical bone area. Thus, our model system highlights the regulatory complexity of osteogenic differentiation and identifies novel osteogenic genes.
-
Stem Cells and Developmental Biology
In STAR Protocols on 17 June 2022 by Nano, R., Sim, S. L., et al.
A need to identify a stem cell source for human endothelial colony forming cells (ECFCs) and mesenchymal stem cells (MSCs) that is high yield is crucial for their implementation in ischemia. Our lab has developed an isolation protocol to do this using full-term human villous placental tissue. This protocol describes enzymatic tissue digestion followed by MACS and FACS, achieving an 8 times greater yield versus traditional isolation techniques and delivering pure fetal stem cell colonies within 21-28 days cell culture. For complete details on the use and execution of this protocol, please refer to Patel et al. (2013) and Patel et al. (2014).
© 2022 The Author(s).
-
Stem Cells and Developmental Biology
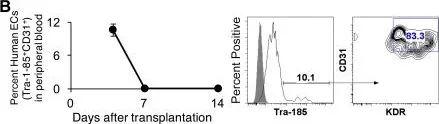
In Stem Cells Transl Med on 1 March 2017 by Gori, J. L., Butler, J. M., et al.
Fig.2.B

-
FC/FACS
-
Macaca mulatta (Rhesus Monkey)
Collected and cropped from Stem Cells Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 1