Maintenance of genome integrity is instrumental in preventing cancer. In addition to DNA repair pathways that prevent damage to DNA, damage tolerance pathways allow for the survival of cells that encounter DNA damage during replication. The Rad6/18 pathway is instrumental in this process, mediating damage bypass by ubiquitination of proliferating cell nuclear antigen. Previous studies have shown different roles of Rad18 in vivo and in tumorigenesis. Here, we show that B cells induce Rad18 expression upon proliferation induction. We have therefore analysed the role of Rad18 in B cell activation as well as in B cell lymphomagenesis mediated by an Eµ-Myc transgene. We find no activation defects or survival differences between Rad18 WT mice and two different models of Rad18 deficient tumour mice. Also, tumour subtypes do not differ between the mouse models. Accordingly, functions of Rad18 in B cell activation and tumorigenesis may be compensated for by other pathways in B cells.
© 2024. The Author(s).
Product Citations: 10
Role of Rad18 in B cell activation and lymphomagenesis.
In Scientific Reports on 25 March 2024 by Kalweit, K., Gölling, V., et al.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Nature Communications on 19 April 2022 by Fleig, S., Kapanadze, T., et al.
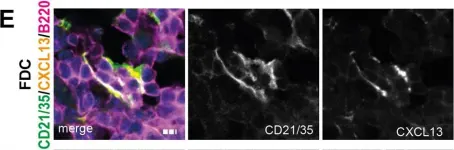
Tertiary lymphoid structures (TLS) are lymph node-like immune cell clusters that emerge during chronic inflammation in non-lymphoid organs like the kidney, but their origin remains not well understood. Here we show, using conditional deletion strategies of the canonical Notch signaling mediator Rbpj, that loss of endothelial Notch signaling in adult mice induces the spontaneous formation of bona fide TLS in the kidney, liver and lung, based on molecular, cellular and structural criteria. These TLS form in a stereotypical manner around parenchymal arteries, while secondary lymphoid structures remained largely unchanged. This effect is mediated by endothelium of blood vessels, but not lymphatics, since a lymphatic endothelial-specific targeting strategy did not result in TLS formation, and involves loss of arterial specification and concomitant acquisition of a high endothelial cell phenotype, as shown by transcriptional analysis of kidney endothelial cells. This indicates a so far unrecognized role for vascular endothelial cells and Notch signaling in TLS initiation.
© 2022. The Author(s).
-
IHC
-
IHC-IF
-
Mus musculus (House mouse)
Impact of Chk1 dosage on somatic hypermutation in vivo.
In Immunology and Cell Biology on 1 September 2021 by Bello, A. & Jungnickel, B.
Checkpoint signaling in the context of a functional DNA damage response is crucial for the prevention of oncogenic transformation of cells. Our immune system, though, takes the risk of attenuated checkpoint responses during immunoglobulin diversification. B cells undergo continuous DNA damage and error-prone repair of their immunoglobulin genes during the process of somatic hypermutation. An accompanying attenuation of the DNA damage response via the ATR-Chk1 axis in B cells is believed to allow for a better DNA damage tolerance and for evasion of apoptosis, so as to ensure mutations to be passed on. We sought to determine whether the downregulation of Chk1 could also directly influence the process of hypermutation in vivo by altering the relative activity of error-prone DNA repair pathways. We analyzed the humoral response and the hypermutation process in mice whose B cells express reduced levels of the Chk1 protein. We found that Chk1 heterozygosity limits the accumulation of mutations in the immunoglobulin loci, likely by impacting on the survival of B cells as they accumulate DNA damage. Nevertheless, we unveiled an unanticipated role for Chk1 downregulation in favoring A/T mutagenesis at the antibody-variable regions during hypermutation. Even though immunoglobulin mutagenesis was found to be reduced, Chk1 signaling attenuation allows for sustained mutagenesis outside the immunoglobulin loci. Our study thus reveals that a proper Chk1 dosage is crucial for adequate somatic hypermutation in B cells.
© 2021 The Authors. Immunology & Cell Biology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
In The Journal of Immunology on 15 September 2019 by Hirth, G., Svensson, C. M., et al.
During somatic hypermutation (SHM) of Ig genes in germinal center B cells, lesions introduced by activation-induced cytidine deaminase are processed by multiple error-prone repair pathways. Although error-free repair by homologous recombination (HR) is crucial to prevent excessive DNA strand breakage at activation-induced cytidine deaminase off-target genes, its role at the hypermutating Ig locus in the germinal center is unexplored. Using B cell-specific inactivation of the critical HR factor Brca2, we detected decreased proliferation, survival, and thereby class switching of ex vivo-activated B cells. Intriguingly, an HR defect allowed for a germinal center reaction and affinity maturation in vivo, albeit at reduced amounts. Analysis of SHM revealed that a certain fraction of DNA lesions at C:G bp was indeed repaired in an error-free manner via Brca2 instead of being processed by error-prone translesion polymerases. By applying a novel pseudo-time in silico analysis of mutational processes, we found that the activity of A:T mutagenesis during SHM increased during a germinal center reaction, but this was in part defective in Brca2-deficient mice. These mutation pattern changes in Brca2-deficient B cells were mostly specific for the Ig V region, suggesting a local or time-dependent need for recombination repair to survive high rates of SHM and especially A:T mutagenesis.
Copyright © 2019 by The American Association of Immunologists, Inc.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Molecular Cell on 15 November 2018 by Delgado-Benito, V., Rosen, D. B., et al.
Class switch recombination (CSR) is a DNA recombination reaction that diversifies the effector component of antibody responses. CSR is initiated by activation-induced cytidine deaminase (AID), which targets transcriptionally active immunoglobulin heavy chain (Igh) switch donor and acceptor DNA. The 3' Igh super-enhancer, 3' regulatory region (3'RR), is essential for acceptor region transcription, but how this function is regulated is unknown. Here, we identify the chromatin reader ZMYND8 as an essential regulator of the 3'RR. In B cells, ZMYND8 binds promoters and super-enhancers, including the Igh enhancers. ZMYND8 controls the 3'RR activity by modulating the enhancer transcriptional status. In its absence, there is increased 3'RR polymerase loading and decreased acceptor region transcription and CSR. In addition to CSR, ZMYND8 deficiency impairs somatic hypermutation (SHM) of Igh, which is also dependent on the 3'RR. Thus, ZMYND8 controls Igh diversification in mature B lymphocytes by regulating the activity of the 3' Igh super-enhancer.Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
In Nat Commun on 19 April 2022 by Fleig, S., Kapanadze, T., et al.
Fig.2.E

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1