Spark-discharged anodic oxidation coating on commercially pure titanium (SAc.p.Ti) has been shown to promote bone conduction and bone matrix mineralization during new bone formation. This study hypothesized that the combination of SAc.p.Ti with dental pulp stem cells (DPSCs) would enhance new bone formation. The objective was to evaluate the effect of this combination in a rat bone defect model.
DPSCs were isolated from Sprague-Dawley (SD) rat incisors and cultured. Calvarial bone defects were created in SD rats, followed by transplantation of commercially pure titanium (c.p.Ti), SAc.p.Ti, or SAc.p.Ti combined with DPSCs. Bone formation was assessed using micro-computed tomography (micro-CT). Toluidine blue O staining was employed to evaluate bone-implant contact and the newly formed bone area. Hematoxylin-eosin (HE) staining was performed to identify osteoblast-like cells.
Micro-CT analysis revealed hard tissue formation on the surface of SAc.p.Ti. Toluidine blue O staining showed significantly greater bone-implant contact and newly formed bone area in the SAc.p.Ti/DPSC group compared to the c.p.Ti and SAc.p.Ti groups. HE staining confirmed the presence of osteoblast-like cells at the defect margins, with evidence of new bone formation on the surface of SAc.p.Ti and in the SAc.p.Ti/DPSC groups.
The combination of SAc.p.Ti and DPSCs presents a promising strategy for promoting new bone formation in rat calvarial defect model.
Product Citations: 35
In Journal of Prosthodontic Research on 11 February 2025 by Kobayashi, T., Hata, M., et al.
-
Rattus norvegicus (Rat)
-
Stem Cells and Developmental Biology
In Cell Stem Cell on 5 September 2024 by Chen, R., Fan, R., et al.
Embryonic diapause is a reproductive adaptation that enables some mammalian species to halt the otherwise continuous pace of embryonic development. In this dormant state, the embryo exploits poorly understood regulatory mechanisms to preserve its developmental potential for prolonged periods of time. Here, using mouse embryos and single-cell RNA sequencing, we molecularly defined embryonic diapause at single-cell resolution, revealing transcriptional dynamics while the embryo seemingly resides in a state of suspended animation. Additionally, we found that the dormant pluripotent cells rely on integrin receptors to sense their microenvironment and preserve their viability via Yap/Taz-mediated prosurvival signaling.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Stem Cells and Developmental Biology
In Obesity (Silver Spring, Md.) on 1 September 2023 by Lv, Y., Xia, F., et al.
Visceral obesity contributes to obesity-related complications; however, the intrinsic mechanism of depot-specific adipose tissue behavior remains unclear. Despite the pro-adipogenesis role of glucocorticoids (GCs) in adipogenesis, the role of GCs in visceral adiposity rather than in subcutaneous adipose tissue is not established. Because adipocyte progenitors display a striking depot-specific pattern, the regulatory pathways of novel progenitor subtypes within different depots remain unclear. This study describes a cell-specific mechanism underlying visceral adiposity.
A diverse panel of novel depot-specific adipose progenitors was screened in mice and human samples. The transcriptome distinction and various responses of novel progenitor subtypes of GCs were further measured using the GC receptor-chromatin immunoprecipitation assay and RNA sequencing. The mechanism of novel subtypes was identified using transposase-accessible chromatin analysis and bisulfite sequencing and further confirmed using precise editing of CpG methylation.
Platelet-derived growth factor receptor α (PDGFRα+ ) progenitors, which were dominant in the visceral adipose tissue, were GC-sensitive beige adipose progenitors, whereas CD137+ progenitors, which were dominant in the subcutaneous adipose tissue, were GC-passive beige adipose progenitors. Expression of miR-27b, an inhibitor of adipocyte browning, was significantly increased in PDGFRα+ progenitors treated with GCs. Using transposase-accessible chromatin analysis, bisulfite sequencing, and precise editing of CpG methylation, TEA domain transcription factor 1 (TEAD1) was discovered to be uniquely hypomethylated in PDGFRα+ progenitors.
GCs inhibited the PDGFRα+ progenitors' browning process via miR-27b, which was transcriptionally activated by the collaboration of TEAD1 with the GC receptor. These data provide insights into the mechanism of depot-specific variations in high-fat diet-induced obesity.
© 2023 The Obesity Society.
-
FC/FACS
In Molecular Medicine Reports on 1 July 2023 by Yang, F., Wu, M., et al.
In the field of orthopedics, defects in large bones have proven challenging to resolve. The aim of the present study was to address this problem through the combination of tantalum metal (pTa) with exosomes derived from bone marrow mesenchymal stem cells (BMSCs), which have the potential to enhance regeneration of full thickness femoral bone defects in rats. Cell culture results demonstrated that exosomes improved the proliferation and differentiation of BMSCs. Following establishment of a supracondylar femoral bone defect, exosomes and pTa were implanted into the defect area. Results demonstrated that pTa acts as a core scaffold for cell adhesion and exhibits good biocompatibility. Moreover, micro‑CT scan results as well as histological examination demonstrated that pTa had a significant effect on osteogenesis, with the addition of exosomes further promoting bone tissue regeneration and repair. In conclusion, this novel composite scaffold can effectively promote bone regeneration in large bone defect areas, providing a new approach for the treatment of large bone defects.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Biochemistry and Molecular biology
Polarity inversion reorganizes the stem cell compartment of the trophoblast lineage.
In Cell Reports on 25 April 2023 by Ozguldez, H. O., Govindasamy, N., et al.
The extra-embryonic tissues that form the placenta originate from a small population of trophectoderm cells with stem cell properties, positioned at the embryonic pole of the mouse blastocyst. During the implantation stages, the polar trophectoderm rapidly proliferates and transforms into extra-embryonic ectoderm. The current model of trophoblast morphogenesis suggests that tissue folding reshapes the trophoblast during the blastocyst to egg cylinder transition. Instead of through folding, here we found that the tissue scale architecture of the stem cell compartment of the trophoblast lineage is reorganized via inversion of the epithelial polarity axis. Our findings show the developmental significance of polarity inversion and provide a framework for the morphogenetic transitions in the peri-implantation trophoblast.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
IHC-IF
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
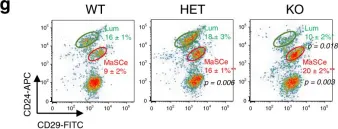
In Nat Commun on 15 September 2020 by Kim, M. R., Wu, M. J., et al.
Fig.1.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
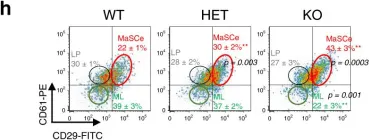
In Nat Commun on 15 September 2020 by Kim, M. R., Wu, M. J., et al.
Fig.1.H
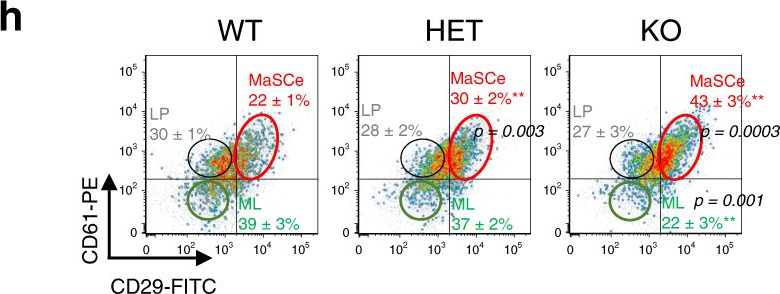
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
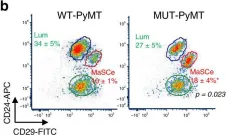
In Nat Commun on 15 September 2020 by Kim, M. R., Wu, M. J., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
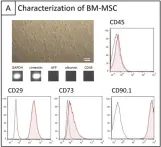
In PLoS One on 23 July 2013 by Boeykens, N., Ponsaerts, P., et al.
Fig.3.A

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4