Platelets enhance coagulation by exposing phosphatidylserine (PS) on their cell surface in response to strong agonist activation. Transient receptor potential channels, including TRPC6, have been implicated in the calcium influx central to this process. Here, we characterize the effect of a Trpc6 gain-of-function (GOF) disease-associated, and a dominant negative (DN), mutation on murine platelet activation. Platelets from mice harboring Trpc6E896K/E896K (GOF) and Trpc6DN/DN mutations were subject to in vitro analysis. Trpc6E896K/E896K and Trpc6DN/DN mutant platelets show enhanced and absent calcium influx, respectively, upon addition of the TRPC3/6 agonist GSK1702934A (GSK). GSK was sufficient to induce integrin αIIbβ3 activation, P-selection and PS exposure, talin cleavage, and MLC2 phosphorylation in Trpc6E896K/E896K, but not in wild-type, platelets. Thrombin-induced calcium influx and PS exposure were enhanced, and clot retraction delayed, by GOF TRPC6, while no differences were noted between wild-type and Trpc6DN/DN platelets. In contrast, Erk activation upon GSK treatment was absent in Trpc6DN/DN, and enhanced in Trpc6E896K/E896K, platelets, compared to wild-type. The positive allosteric modulator, TRPC6-PAM-C20, and fluoxetine maintained their ability to enhance and inhibit, respectively, GSK-mediated calcium influx in Trpc6E896K/E896K platelets. The data demonstrate that gain-of-function mutant TRPC6 channel can enhance platelet activation, including PS exposure, while confirming that TRPC6 is not necessary for this process. Furthermore, the results suggest that Trpc6 GOF disease mutants do not simply increase wild-type TRPC6 responses, but can affect pathways not usually modulated by TRPC6 channel activity, displaying a true gain-of-function phenotype.
Product Citations: 13
Trpc6 gain-of-function disease mutation enhances phosphatidylserine exposure in murine platelets.
In PLoS ONE on 25 June 2022 by Boekell, K. L., Brown, B. J., et al.
-
FC/FACS
-
Mus musculus (House mouse)
Preprint on BioRxiv : the Preprint Server for Biology on 3 February 2022 by Boekell, K. L., Brown, B. J., et al.
Platelets enhance coagulation by exposing phosphatidylserine (PS) on their cell surface in response to strong agonist activation. Transient receptor potential channels, including TRPC6, have been implicated in the calcium influx central to this process. Here, we characterize the effect of a Trpc6 gain-of-function (GOF) disease-associated, and a dominant negative (DN), mutation on murine platelet activation. Platelets from mice harboring Trpc6 E896K/E896K (GOF) and Trpc6 DN/DN mutations were subject to in vitro analysis. Trpc6 E896K/E896K and Trpc6 DN/DN mutant platelets show enhanced and absent calcium influx, respectively, upon addition of the TRPC3/6 agonist GSK1702934A (GSK). GSK was sufficient to induce integrin αIIbβ3 activation, P-selection and PS exposure, talin cleavage, and MLC2 phosphorylation in Trpc6 E896K/E896K , but not in wild-type, platelets. Thrombin-induced calcium influx and PS exposure were enhanced, and clot retraction delayed, by GOF TRPC6, while no differences were noted between wild-type and Trpc6 DN/DN platelets. In contrast, Erk activation upon GSK treatment was absent in Trpc6 DN/DN , and enhanced in Trpc6 E896K/E896K , platelets, compared to wild-type. The positive allosteric modulator, TRPC6-PAM-C20, and fluoxetine maintained their ability to enhance and inhibit, respectively, GSK-mediated calcium influx in Trpc6 E896K/E896K platelets. The data demonstrate that gain-of-function mutant TRPC6 channel can enhance platelet activation, including PS exposure, while confirming that TRPC6 is not necessary for this process. Furthermore, they suggest that Trpc6 GOF disease mutants do not simply increase wild-type TRPC6 responses, but can affect pathways not usually modulated by TRPC6 channel activity, displaying a true gain-of-function phenotype.
-
FC/FACS
-
Mus musculus (House mouse)
Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis.
In Nature Communications on 27 January 2022 by Fielding, C., García-García, A., et al.
The sympathetic nervous system has been evolutionary selected to respond to stress and activates haematopoietic stem cells via noradrenergic signals. However, the pathways preserving haematopoietic stem cell quiescence and maintenance under proliferative stress remain largely unknown. Here we found that cholinergic signals preserve haematopoietic stem cell quiescence in bone-associated (endosteal) bone marrow niches. Bone marrow cholinergic neural signals increase during stress haematopoiesis and are amplified through cholinergic osteoprogenitors. Lack of cholinergic innervation impairs balanced responses to chemotherapy or irradiation and reduces haematopoietic stem cell quiescence and self-renewal. Cholinergic signals activate α7 nicotinic receptor in bone marrow mesenchymal stromal cells leading to increased CXCL12 expression and haematopoietic stem cell quiescence. Consequently, nicotine exposure increases endosteal haematopoietic stem cell quiescence in vivo and impairs hematopoietic regeneration after haematopoietic stem cell transplantation in mice. In humans, smoking history is associated with delayed normalisation of platelet counts after allogeneic haematopoietic stem cell transplantation. These results suggest that cholinergic signals preserve stem cell quiescence under proliferative stress.
© 2022. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In British Journal of Pharmacology on 1 January 2022 by Jiang, L. P., Ji, J. Z., et al.
Overweight or obese patients exhibit poorer platelet responses to clopidogrel. However, the mechanisms behind this phenotype remain to be elucidated. Here, we sought to discover whether and why obesity could affect the metabolic activation of and/or platelet response to clopidogrel in obese patients and high-fat diet-induced obese mice.
A post hoc stratified analysis of an observational clinical study was performed to investigate changes in residual platelet reactivity with increasing body weight in patients taking clopidogrel. Furthermore, high-fat diet-induced obese mice were used to reveal alterations in systemic exposure of clopidogrel thiol active metabolite H4, ADP-induced platelet activation and aggregation, the expression of genes involved in the metabolic activation of clopidogrel, count of circulating reticulated and mature platelets, and proliferation profiles of megakaryocytes in bone marrow. The relevant genes and potential signalling pathways were predicted and enriched according to the GEO datasets available from obese patients.
Obese patients exhibited significantly attenuated antiplatelet effects of clopidogrel. In diet-induced obese mice, systemic exposure of clopidogrel active metabolite H4 was reduced but that of its hydrolytic metabolite was increased due to down-regulation of certain P450s but up-regulation of carboxylesterase-1 in the liver. Moreover, enhanced proliferation of megakaryocytes and elevated platelet count also contributed.
Obesity attenuated metabolic activation of clopidogrel and increased counts of circulating reticulated and mature platelets, leading to impaired platelet responsiveness to the drug in mice, suggesting that clopidogrel dosage may need to be adjusted adequately in overweight or obese patients.
© 2021 The British Pharmacological Society.
-
Biochemistry and Molecular biology
-
Pharmacology
Methylation of dual-specificity phosphatase 4 controls cell differentiation.
In Cell Reports on 27 July 2021 by Su, H., Jiang, M., et al.
Mitogen-activated protein kinases (MAPKs) are inactivated by dual-specificity phosphatases (DUSPs), the activities of which are tightly regulated during cell differentiation. Using knockdown screening and single-cell transcriptional analysis, we demonstrate that DUSP4 is the phosphatase that specifically inactivates p38 kinase to promote megakaryocyte (Mk) differentiation. Mechanistically, PRMT1-mediated methylation of DUSP4 triggers its ubiquitinylation by an E3 ligase HUWE1. Interestingly, the mechanistic axis of the DUSP4 degradation and p38 activation is also associated with a transcriptional signature of immune activation in Mk cells. In the context of thrombocytopenia observed in myelodysplastic syndrome (MDS), we demonstrate that high levels of p38 MAPK and PRMT1 are associated with low platelet counts and adverse prognosis, while pharmacological inhibition of p38 MAPK or PRMT1 stimulates megakaryopoiesis. These findings provide mechanistic insights into the role of the PRMT1-DUSP4-p38 axis on Mk differentiation and present a strategy for treatment of thrombocytopenia associated with MDS.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
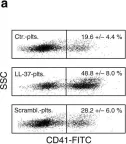
In Nat Commun on 18 April 2018 by Pircher, J., Czermak, T., et al.
Fig.5.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1