Immune checkpoint inhibitors have revolutionized cancer therapy, but many patients fail to respond or develop resistance, often due to reduced T cell activity. Costimulation via 4-1BB has emerged as a promising approach to enhance the effector function of antigen-primed T cells. Bispecific T cell-engaging (TCE) antibodies are an effective way to provide tumor-specific T cell receptor-mediated signaling to tumor-infiltrating lymphocytes. mRNA-based delivery of bispecific antibodies, offer a novel approach to enhance tumor-specific immune responses while minimizing adverse effects.
Two bispecific antibodies were generated: the EGFR x CD3 TCE antibody (LiTE) and the PD-L1 x 4-1BB costimulatory antibody (LiTCo), which was further fused to a high FcRn albumin variant (Albu-LiTCo). The mRNA encoding these bispecific antibodies contains an N1-methylpseudouridine modified nucleoside and regulatory sequences to ensure proper expression and stability. A series of in vitro assays and cell-based analyses were performed to characterize both antibodies. The in vivo efficacy of the mRNA-encoded bispecific antibodies was evaluated in xenograft tumor models expressing EGFR.
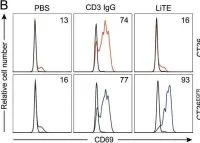
We investigated the combined effect of two mRNA-encoded Fc-free bispecific antibodies with complementary mechanisms of action: an EGFR-targeting TCE and a half-life extended PD-L1 x 4-1BB costimulatory antibody. The mRNAs encoding both bispecific LiTERNA and Albu-LiTCoRNA, showed similar binding specificity and in vitro function to their protein analogues. Pharmacokinetic studies demonstrated sustained expression of both bispecific antibodies following intravenous administration of the mRNAs formulated using a polymer/lipid-based nanoparticle (LNP) but different pharmacokinetic profiles, shorter for the TCE and longer for the PD-L1 x 4-1BB. When administered as a mRNA-LNP combination (ComboRNA), the growth of EGFR-positive tumors in immunocompetent mice was significantly inhibited, resulting in tumor regression in 20% of cases with no associated toxicity. Histological analysis confirmed increased T cell infiltration in the tumors treated with LITERNA and ComboRNA. Repeated administration resulted in sustained production of bispecific antibodies with different exposure cycles and potent antitumor activity with a favorable safety profile.
These results highlight the potential of combining two mRNA-encoded bispecific antibodies with different mechanisms of action and programmable half-life for cancer immunotherapy.
Copyright © 2025 Hangiu, Navarro, Frago, Rubio-Pérez, Tapia-Galisteo, Díez-Alonso, Gómez-Rosel, Silva-Pilipich, Vanrell, Smerdou, Howard, Sanz, Álvarez-Vallina and Compte.
Product Citations: 3
In Frontiers in Immunology on 21 January 2025 by Hangiu, O., Navarro, R., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In Journal of Neuroinflammation on 10 October 2022 by Wu, X., Shen, Q., et al.
The immune system has been implicated in synaptic plasticity, inflammation, and the progression of Alzheimer's disease (AD). However, there were few studies on improving the niche microenvironment of neural stem cells (NSCs) in the brain of AD to promote adult hippocampal neurogenesis (AHN) by regulating the function of non-parenchymal immune cells.
The lymph nodes of amyloid precursor protein/presenilin 1 (APP/PS1) and 3xTg (APP/PS1/tau) mouse models of AD were treated with photobiomodulation therapy (PBMT) for 10 J/cm2 per day for 1 month (10 min for each day), T lymphocytes isolated from these two AD models were treated with PBMT for 2 J/cm2 (5 min for each time). The NSCs isolated from hippocampus of these two AD models at E14, and the cells were co-cultivated with PBMT-treated T lymphocyte conditioned medium for NSCs differentiation.
Our results showed that PBMT treatment could promote AHN and reverse cognitive deficits in AD mouse model. The expression of interferon-γ (IFN-γ) and interleukin-10 (IL-10) was upregulated in the brain of these two AD models after PBMT treated, which was induced by the activation of Janus kinase 2 (JAK2)-mediated signal transducer and activator of transcription 4 (STAT4)/STAT5 signaling pathway in CD4+ T cells. In addition, elevated CD4+ T cell levels and upregulated transforming growth factor-β1 (TGFβ1)/insulin-like growth factors-1 (IGF-1)/brain-derived neurotrophic factor (BDNF) protein expression levels were also detected in the brain. More importantly, co-cultivated the PBMT-treated T lymphocyte conditioned medium with NSCs derived from these two AD models was shown to promote NSCs differentiation, which was reflected in the upregulation of both neuronal class-III β-tubulin (Tuj1) and postsynaptic density protein 95 (PSD95), but the effects of PBMT was blocked by reactive oxygen species (ROS) scavenger or JAK2 inhibitor.
Our research suggests that PBMT exerts a beneficial neurogenesis modulatory effect through activating the JAK2/STAT4/STAT5 signaling pathway to promote the expression of IFN-γ/IL-10 in non-parenchymal CD4+ T cells, induction of improvement of brain microenvironmental conditions and alleviation of cognitive deficits in APP/PS1 and 3xTg-AD mouse models.
© 2022. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Neuroscience
In Frontiers in Immunology on 13 September 2016 by Akk, A., Springer, L. E., et al.
Paramyxoviral infection in childhood has been linked to a significant increased rate of asthma development. In mice, paramyxoviral infection with the mouse parainfluenza virus type I, Sendai virus (Sev), causes a limited bronchiolitis followed by persistent asthma traits. We have previously shown that the absence of cysteine protease dipeptidyl peptidase I (DPPI) dampened the acute lung inflammatory response and the subsequent asthma phenotype induced by Sev. Adoptive transfer of wild-type neutrophils into DPPI-deficient mice restored leukocyte influx, the acute cytokine response, and the subsequent mucous cell metaplasia that accompanied Sev-induced asthma phenotype. However, the exact mechanism by which DPPI-sufficient neutrophils promote asthma development following Sev infection is still unknown. We hypothesize that neutrophils recruited to the alveolar space following Sev infection elaborate neutrophil extracellular traps (NETs) that propagate the inflammatory cascade, culminating in the eventual asthma phenotype. Indeed, we found that Sev infection was associated with NET formation in the lung and release of cell-free DNA complexed to myeloperoxidase in the alveolar space and plasma that peaked on day 2 post infection. Absence of DPPI significantly attenuated Sev-induced NET formation in vivo and in vitro. Furthermore, concomitant administration of DNase 1, which dismantled NETs, or inhibition of peptidylarginine deiminase 4 (PAD4), an essential mediator of NET formation, suppressed the early inflammatory responses to Sev infection. Lastly, NETs primed bone marrow-derived cells to release cytokines that can amplify the inflammatory cascade.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Front Immunol on 21 January 2025 by Hangiu, O., Navarro, R., et al.
Fig.1.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1