Modified Xi-Jiao-Di-Huang decoction (MXJDH) has significant clinical efficacy for the treatment of sepsis; however, its mechanism of action remains unclear. The purpose of this study was to investigate the protective effects of MXJDH in septic mice and explore its mechanism of action.
Utilizing UPLC-Q-TOF-MS, we identified the primary constituents of the compound MXJDH. Subsequently, we created a mouse model for sepsis, observing their overall condition, including specific symptoms and behavior. We also monitored key inflammatory markers and pathological changes in their organs. Flow cytometry was then employed to assess the polarization of macrophages. Transcriptome sequencing was used to identify genes with altered expression patterns. We investigated the connection between MXJDH and the Pim2/NF-κB signaling pathway, a crucial regulatory mechanism in inflammation. Finally, we examined the expression and tissue distribution of macrophages in the sepsis-induced mice.
MXJDH effectively reduces inflammation in sepsis mice, leading to a progressive recovery of organ functions. Moreover, MXJDH facilitates the conversion of macrophages from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype. This transformation is potentially mediated through the Pim2/NF-κB signaling pathway. By suppressing Pim2 expression, MXJDH mitigates the nuclear translocation of NF-κB, thereby modulating the expression of downstream inflammatory mediators. The role of MXJDH in regulating macrophage polarization has also been confirmed in sepsis mouse tissues.
MXJDH regulates macrophage polarization, inhibits CRS, and alleviates sepsis by inhibiting the Pim2/NF-κB signaling pathway.
© 2025 Ge et al.
Product Citations: 36
In Journal of Inflammation Research on 18 April 2025 by Ge, F., Tian, F., et al.
-
Immunology and Microbiology
In Journal of Cellular and Molecular Medicine on 1 March 2025 by Zeng, L., Zhang, J., et al.
The lipopolysaccharide-induced acute lung injury (ALI) mouse model is used to simulate human acute respiratory distress syndrome (ARDS), which has a high mortality rate. An imbalance between M1 and M2 macrophages, characterised by an increase in M1 macrophages, was observed in sepsis-induced ALI. We report that laminarin, an active ingredient found in algae, exhibits exceptional performance in a mouse model of sepsis-induced ALI. It ameliorates lung edema, enhances the survival rate of mice and reduces the levels of the inflammatory factors TNF-α and IL-6. Furthermore, laminarin reduced the expression of CD86, which are markers associated with M1 macrophages. Laminarin treatment reduces the secretion of TNF-α and IL-6 in LPS-stimulated macrophages. Laminarin treatment also decreases glucose uptake in LPS-stimulated macrophages. Transcriptome sequencing reveals that genes downregulated in LPS-stimulated macrophages following laminarin treatment are predominantly enriched in the HIF-1α signalling pathway. Experimental validation confirms that laminarin treatment of LPS-stimulated macrophages reduces the expression of HIF-1α and significantly decreases the expression of related indicators ROS and NLRP3. After using siRNA to knock down HIF-1α in RAW264.7 cells, the inhibitory effect of laminarin on LPS-induced M1 polarisation of macrophages is abolished. This suggests that laminarin may potentially inhibit macrophage polarisation towards the M1 phenotype by downregulating the HIF-1α signal. In conclusion, the data presented in our study demonstrate that laminarin can effectively reduce M1 macrophage polarisation by downregulating HIF-1α signalling. This makes it a novel candidate drug for the treatment of LPS-induced ALI.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
New insights into allergic rhinitis treatment: MSC nanovesicles targeting dendritic cells.
In Journal of Nanobiotechnology on 19 September 2024 by Liu, J., Wang, M., et al.
Allergic rhinitis (AR) is a condition with limited treatment options. This study investigates the potential use of mesenchymal stem cell (MSC) nanovesicles as a novel therapy for AR. Specifically, the study explores the underlying mechanisms of MSC nanovesicle therapy by targeting dendritic cells (DCs). The researchers fabricated DC-targeted P-D2-EVs nanovesicles and characterized their properties. Transcriptomic sequencing and single-cell sequencing analyses were performed to study the impact of P-D2-EVs on AR mice, identifying core genes involved in the treatment. In vitro cell experiments were conducted to validate the effects of P-D2-EVs on DC metabolism, Th2 differentiation, and ILC2 activation. The results showed that P-D2-EVs efficiently targeted DCs. Transcriptomic sequencing analysis revealed differential expression of 948 genes in nasal tissue DCs of mice treated with P-D2-EVs. Single-cell sequencing further revealed that P-D2-EVs had inhibitory effects on DC activation, Th2 differentiation, and ILC2 activation, with Fut1 identified as the core gene. Validation experiments demonstrated that P-D2-EVs improved IL10 metabolism in DCs by downregulating Fut1 expression, thereby suppressing Th2 differentiation and ILC2 activation. Animal experiments confirmed the inhibitory effects of P-D2-EVs and their ability to ameliorate AR symptoms in mice. The study suggests that P-D2-EVs reshape DC metabolism and suppress Th2 differentiation and ILC2 activation through the inhibition of the Fut1/ICAM1/P38 MAPK signaling pathway, providing a potential therapeutic approach for AR.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In JCI Insight on 22 August 2024 by Rosberg, R., Smoląg, K. I., et al.
Glioblastoma (GBM) is the most aggressive form of glioma with a high rate of relapse despite intensive treatment. Tumor recurrence is tightly linked to radio-resistance, which in turn is associated with hypoxia. Here, we discovered a strong link between hypoxia and local complement signaling using publicly available bulk, single-cell, and spatially resolved transcriptomic data from patients with GBM. Complement component 3 (C3) and the receptor C3AR1 were both associated with aggressive disease and shorter survival in human glioma. In a genetically engineered mouse model of GBM, we found C3 specifically in hypoxic tumor areas. In vitro, we found an oxygen level-dependent increase in C3 and C3AR1 expression in response to hypoxia in several GBM and stromal cell types. C3a induced M2 polarization of cultured microglia and macrophages in a C3aR-dependent fashion. Targeting C3aR using the antagonist SB290157 prolonged survival of glioma-bearing mice both alone and in combination with radiotherapy while reducing the number of M2-polarized macrophages. Our findings establish a strong link between hypoxia and complement pathways in GBM and support a role of hypoxia-induced C3a/C3aR signaling as a contributor to glioma aggressiveness by regulating macrophage polarization.
-
FC/FACS
-
Mus musculus (House mouse)
-
Homo sapiens (Human)
-
Cancer Research
In The EMBO Journal on 1 August 2024 by Liaqat, I., Hilska, I., et al.
Migrating cells preferentially breach and integrate epithelial and endothelial monolayers at multicellular vertices. These sites are amenable to forces produced by the migrating cell and subsequent opening of the junctions. However, the cues that guide migrating cells to these entry portals, and eventually drive the transmigration process, are poorly understood. Here, we show that lymphatic endothelium multicellular junctions are the preferred sites of dendritic cell transmigration in both primary cell co-cultures and in mouse dermal explants. Dendritic cell guidance to multicellular junctions was dependent on the dendritic cell receptor CCR7, whose ligand, lymphatic endothelial chemokine CCL21, was exocytosed at multicellular junctions. Characterization of lymphatic endothelial secretory routes indicated Golgi-derived RAB6+ vesicles and RAB3+/27+ dense core secretory granules as intracellular CCL21 storage vesicles. Of these, RAB6+ vesicles trafficked CCL21 to the multicellular junctions, which were enriched with RAB6 docking factor ELKS (ERC1). Importantly, inhibition of RAB6 vesicle exocytosis attenuated dendritic cell transmigration. These data exemplify how spatially-restricted exocytosis of guidance cues helps to determine where dendritic cells transmigrate.
© 2024. The Author(s).
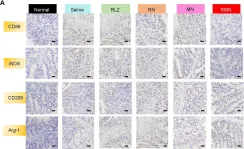
In Theranostics on 16 September 2020 by Sun, T., Kwong, C. H. T., et al.
Fig.5.A

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 1