Intervertebral disc degeneration (IDD) is characterized by oxidative-stress driven progressive apoptosis and senescence of nucleus pulposus mesenchymal stem cells (NP-MSCs). MOTS-c, a 16-amino acid peptide encoded by the mitochondrial 12S rRNA open reading frame, has emerged as a key regulator of cellular metabolism, oxidative stress, and senescence. This study investigated the therapeutic potential of MOTS-c in countering tert-butyl hydroperoxide (TBHP)-induced oxidative damage in NP-MSCs, and we developed a novel biomaterial strategy for IDD treatment.Key findings include.
MOTS-c significantly attenuated TBHP-induced NP-MSC apoptosis (Annexin V+/PI + cells reduced by 48 %, p < 0.001), senescence (SA-β-gal + cells decreased by 52 %, p < 0.005), and ROS overproduction (35 % reduction, p < 0.0001) via activation of the AMPK/SIRT1 pathway. Pharmacological inhibition of SIRT1 abolished these protective effects, confirming pathway specificity.
A sustained-release MOTS-c delivery system (RAD/RMOTS-c) was engineered by conjugating MOTS-c to the self-assembling RADA16-I peptide. The hydrogel exhibited a β-sheet-rich nanofibrous structure (fiber diameter: 362.6 nm), shear-thinning rheology (viscosity: 131-217 Pa s), and sustained peptide release over 7 days.
RAD/RMOTS-c enhanced NP-MSC viability (1.8-fold vs. control, p < 0.005) and extracellular matrix (ECM) synthesis, elevating collagen II/aggrecan expression (2.3-fold, p < 0.05) while suppressing collagen I (63 % reduction, p < 0.001).In Vivo Therapeutic Validation: In a rat IDD model, RAD/RMOTS-c injection preserved disc height (DHI%: 82.4 vs. 58.7 in IDD group, p < 0.001), restored T2-weighted MRI signals (1.5-fold increase, p < 0.001), and reduced histological degeneration scores by 44 % compared to untreated controls (p < 0.001).
This work (1) demonstrates the association between MOTS-c's anti-degenerative effects and AMPK/SIRT1 signaling in NP-MSCs and (2) pioneers a peptide-hydrogel hybrid system that synergistically combines mitochondrial protection with structural support for disc regeneration. The findings can advance IDD therapy toward biology-driven, minimally invasive solutions, aligning with the paradigm of functional biomaterials for degenerative diseases.
© 2025 The Authors.
Product Citations: 34
In Materials Today. Bio on 1 June 2025 by Lin, Y., Yang, R. Y., et al.
-
Stem Cells and Developmental Biology
In International Journal of Molecular Sciences on 7 December 2023 by Lin, Z., Shibuya, Y., et al.
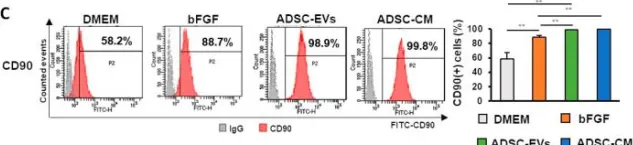
Radiotherapy (RT) is one of three major treatments for malignant tumors, and one of its most common side effects is skin and soft tissue injury. However, the treatment of these remains challenging. Several studies have shown that mesenchymal stem cell (MSC) treatment enhances skin wound healing. In this study, we extracted human dermal fibroblasts (HDFs) and adipose-derived stem cells (ADSCs) from patients and generated an in vitro radiation-induced skin injury model with HDFs to verify the effect of conditioned medium derived from adipose-derived stem cells (ADSC-CM) and extracellular vesicles derived from adipose-derived stem cells (ADSC-EVs) on the healing of radiation-induced skin injury. The results showed that collagen synthesis was significantly increased in wounds treated with ADSC-CM or ADSC-EVs compared with the control group, which promoted the expression of collagen-related genes and suppressed the expression of inflammation-related genes. These findings indicated that treatment with ADSC-CM or ADSC-EVs suppressed inflammation and promoted extracellular matrix deposition; treatment with ADSC-EVs also promoted fibroblast proliferation. In conclusion, these results demonstrate the effectiveness of ADSC-CM and ADSC-EVs in the healing of radiation-induced skin injury.
-
FC/FACS
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In Journal of Immunology Research on 8 November 2023 by Cai, B., Song, W., et al.
Acute lung injury (ALI) is a life-threatening disease that currently lacks a cure. Although stem cell-derived small extracellular vesicles (sEVs) have shown promising effects in the treatment of ALI, their underlying mechanisms and responsible components have yet to be identified. Proprotein convertase subtilisin/kexin type 6 (PCSK6) is a gene involved in inflammation and a potential target of miR-21-5p, a microRNA enriched in stem cell-derived sEVs. The current study investigated the role of PCSK6 in lipopolysaccharide (LPS)-induced ALI and its interaction with miR-21-5p. Notably, our results showed that PCSK6 expression was positively correlated with LPS stimulation. Knockdown of PCSK6 ameliorated LPS-induced inhibition of proliferation and upregulation of permeability in human BEAS-2B cells, whereas PCSK6 overexpression displayed the opposite effects. BEAS-2B cells were able to actively internalize the cocultured bone mesenchymal stem cell (MSC)-derived sEVs (BMSC-sEVs), which alleviated the cell damage caused by LPS. Overexpressing PCSK6, however, eliminated the therapeutic effects of BMSC-sEV coculture. Mechanistically, BMSC-sEVs inhibited PCSK6 expression via the delivery of miR-21-5p, which is directly bound to the PCSK6 gene. Our work provides evidence for the role of PCSK6 in LPS-induced ALI and identified miR-21-5p as a component of BMSC-derived sEVs that suppressed PCSK6 expression and ameliorated LPS-induced cell damage. These results reveal a novel molecular mechanism for ALI pathogenesis and highlight the therapeutic potential of using sEVs released by stem cells to deliver miR-21-5p for ALI treatment.
Copyright © 2023 Bo Cai et al.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Turkish Journal of Biology = Turk Biyoloji Dergisi on 2 August 2023 by Wang, B. Z., Wang, M. M., et al.
Recent clinical developments in tissue bioengineering have applications in acute cardiac ischemia and infarction and include the use of stem cells that combine injectable scaffold material. This study aimed to evaluate the effects of adipose-derived stem cells (ADSCs) that combine the Matrigel scaffold on cardiac morphology/functions. The autologous ADSCs myocardial infarction (MI) model was induced by the permanent ligation method of the left anterior descending coronary artery (LAD). MI-operated rats were randomly divided into PBS group, Matrigel group, PBS plus ADSCs group (PBS+ADSCs), and Matrigel plus ADSCs group (Matrigel+ADSCs). Matrigel was used as an injectable scaffold. Rats with a 1-week-old myocardial infarction were injected with 2 × 106 labeled ADSCs in the border area of the ischemic heart. Heart function was determined by echocardiography. The hemodynamics, cardiac structure, and graft characteristics were evaluated. The ADSCs were successfully isolated and identified, demonstrating a good proliferative status and cell retention in the Matrigel. ADSCs+Matrigel exhibited the most improved heart functions (LVESD, LVEDD, LVFS, LVEF) compared to those of other groups (p < 0.05). ADSCs+Matrigel significantly reduced infarct size compared to other groups (p < 0.05). Cotransplantation of ADSCs and Matrigel showed the best effect on maintaining the thickness of the ventricular wall compared to the other groups (p < 0.05). Engrafted ADSCs played a role in the formation of the neovasculature in myocardial infarction. ADSCs+Matrigel triggered the greatest enhancement in arteriole density than other groups (p < 0.05). Cotransplanting with ADSCs and Matrigel showed significantly higher levels of cardiac troponin T (cTnT), NK2-transcription factor related locus-5 (Nkx2.5), von Willebrand factor (vWF) than the other groups (p < 0.05). In conclusion, this study demonstrated that cotransplanting ADSCs with Matrigel resulted in improved cardiac morphology and cardiac function in the rat model of myocardial infarction.
© TÜBİTAK.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
-
Stem Cells and Developmental Biology
Biomimetic scaffold-based stem cell transplantation promotes lung regeneration.
In Bioengineering Translational Medicine on 1 July 2023 by Wang, L., Feng, M., et al.
Therapeutic options are limited for severe lung injury and disease as the spontaneous regeneration of functional alveolar is terminated owing to the weakness of the inherent stem cells and the dyscrasia of the niche. Umbilical cord mesenchymal-derived stem cells (UC-MSCs) have been applied to clinical trials to promote lung repair through stem cell niche restruction. However, the application of UC-MSCs is hampered by the effectiveness of cell transplantation with few cells homing to the injury sites and poor retention, survival, and proliferation in vivo. In this study, we constructed an artificial three-dimensional (3D) biomimetic scaffold-based MSCs implant to establish a beneficial regeneration niche for endogenous stem cells in situ lung regeneration. The therapeutic potential of 3D biomimetic scaffold-based MSCs implants was evaluated by 3D culture in vitro. And RNA sequencing (RNA-Seq) was mapped to explore the gene expression involved in the niche improvement. Next, a model of partial lung resection was established in rats, and the implants were implanted into the operative region. Effects of the implants on rat resected lung injury repair were detected. The results revealed that UC-MSCs loaded on biomimetic scaffolds exerted strong paracrine effects and some UC-MSCs migrated to the lung from scaffolds and had long-term retention to suppress inflammation and fibrosis in residual lungs and promoted vascular endothelial cells and alveolar type II epithelial cells to enter the scaffolds. Then, under the guidance of the ECM-mimicking structures of scaffolds and the stimulation of the remaining UC-MSCs, vascular and alveolar-like structures were formed in the scaffold region. Moreover, the general morphology of the operative lung was also restored. Taken together, the artificial 3D biomimetic scaffold-based MSCs implants induce in situ lung regeneration and recovery after lung destruction, providing a promising direction for tissue engineering and stem cell strategies in lung regeneration.
© 2023 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
-
Stem Cells and Developmental Biology
In Int J Mol Sci on 7 December 2023 by Lin, Z., Shibuya, Y., et al.
Fig.5.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 1