Aspergillus fumigatus (Af) is a major mould pathogen found ubiquitously in the air. It commonly infects the airways of people with cystic fibrosis (CF) leading to Aspergillus bronchitis or allergic bronchopulmonary aspergillosis. Resident alveolar macrophages and recruited neutrophils are important first lines of defence for clearance of Af in the lung. However, their contribution to the inflammatory phenotype in CF during Af infection is not well understood. Here, utilising CFTR deficient mice we describe a hyperinflammatory phenotype in both acute and allergic murine models of pulmonary aspergillosis. We show that during aspergillosis, CFTR deficiency leads to increased alveolar macrophage death and persistent inflammation of the airways in CF, accompanied by impaired fungal control. Utilising CFTR deficient murine cells and primary human CF cells we show that at a cellular level there is increased activation of NFκB and NFAT in response to Af which, as in in vivo models, is associated with increased cell death and reduced fungal control. Taken together, these studies indicate that CFTR deficiency promotes increased activation of inflammatory pathways, the induction of macrophage cell death and reduced fungal control contributing to the hyper-inflammatory of pulmonary aspergillosis phenotypes in CF.
Copyright: © 2025 Bercusson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Product Citations: 58
In PLoS Pathogens on 1 February 2025 by Bercusson, A., Williams, T. J., et al.
-
Immunology and Microbiology
In STAR Protocols on 20 December 2024 by Wang, X., Wang, Y., et al.
Dendritic cells (DCs) play a central role in the initiation of the adaptive immune response. Here, we present a protocol for isolating and transcriptionally profiling antigen-presenting cells (APCs) from the mouse lung and mediastinal lymph nodes (MLNs) following intranasal immunization. We describe steps for preparing single-cell suspensions from the lung and MLN, along with the detection and RNA sequencing (RNA-seq) of antigen-presenting DCs. This protocol offers a broadly applicable approach for identifying variations in DC subpopulations under diverse experimental conditions. For complete details on the use and execution of this protocol, please refer to Youhui et al.1.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
Eosinophils restrict CRC metastasis by inhibiting pro-tumorigenic SPP1+macrophage differentiation
Preprint on BioRxiv : the Preprint Server for Biology on 17 December 2024 by Handler, K., Raju, D., et al.
Eosinophils, traditionally associated with allergic responses, have emerged as critical immune modulators in colorectal cancer (CRC). Here, we reveal that eosinophils actively shape the tumor microenvironment and influence metastatic progression. Using comprehensive transcriptomics analysis of human CRC and a murine orthotopic tumor model, we identify a conserved tumor-specific eosinophil signature and activation profile. Despite their declining presence in advanced CRC, eosinophils suppress metastatic dissemination by counteracting the pro-tumorigenic functions of SPP1 + macrophages – a subset linked to immune exclusion and tumor metastasis. Mechanistically, eosinophils respond to tumor-derived signals and inhibit macrophage differentiation into SPP1 + cells. Eosinophil depletion exacerbates peritoneal tumor spread. These findings highlight the pivotal role of eosinophils in restraining late-stage CRC progression and unveil a novel eosinophil–macrophage axis as potential therapeutic targets.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 3 December 2024 by Bercusson, A., Williams, T. J., et al.
Aspergillus fumigatus ( Af ) is a major mould pathogen found ubiquitously in the air. It commonly infects the airways of people with cystic fibrosis (CF) leading to Aspergillus bronchitis or allergic bronchopulmonary aspergillosis. Resident alveolar macrophages and recruited neutrophils are important first lines of defence for clearance of Af in the lung. However, their contribution to the inflammatory phenotype in CF during Af infection is not well understood. Here, utilising CFTR deficient mice we describe a hyperinflammatory phenotype in both acute and allergic murine models of pulmonary aspergillosis. We show that during aspergillosis, CFTR deficiency leads to increased alveolar macrophage death and persistent inflammation of the airways in CF, accompanied by impaired fungal control. Utilising CFTR deficient murine cells and primary human CF cells we show that at a cellular level there is increased activation of NFκB and NFAT in response to Af which, as in in vivo models, is associated with increased cell death and reduced fungal control. Taken together, these studies indicate that CFTR deficiency promotes increased activation of inflammatory pathways, the induction of macrophage cell death and reduced fungal control contributing to the hyper-inflammatory of pulmonary aspergillosis phenotypes in CF. Author Summary The airways of people with cystic fibrosis (pwCF) are commonly colonised with Aspergillus fumigatus ( Af ) resulting in a persistent hyperinflammatory state and the development of allergy. Understanding how first line defence innate cells, such as alveolar macrophages and neutrophils, contribute to this hyperinflammatory phenotype is important in developing novel treatments to preserve lung function in pwCF. In this study, we report that CFTR deficiency leads to increased alveolar macrophage death and persistent inflammation of the airways in pwCF, which is associated with impaired control of infection. We identify the increased activation of the transcription factors NFκB and NFAT as the mechanism of increased inflammatory cytokine production in CFTR deficient cells. This work is the first step in describing molecular mechanisms of hyperinflammation in CF in response to fungal infection and lays the groundwork for further dissection of inflammatory pathways to target with immunotherapeutic approaches.
-
Mus musculus (House mouse)
Alveolar macrophage function is impaired following inhalation of berry e-cigarette vapor.
In Proceedings of the National Academy of Sciences of the United States of America on 1 October 2024 by Kulle, A., Li, Z., et al.
In the lower respiratory tract, the alveolar spaces are divided from the bloodstream and the external environment by only a few microns of interstitial tissue. Alveolar macrophages (AMs) defend this delicate mucosal surface from invading infections by regularly patrolling the site. AMs have three behavior modalities to achieve this goal: extending cell protrusions to probe and sample surrounding areas, squeezing the whole cell body between alveoli, and patrolling by moving the cell body around each alveolus. In this study, we found Rho GTPase, cell division control protein 42 (CDC42) expression significantly decreased after berry-flavored e-cigarette (e-cig) exposure. This shifted AM behavior from squeezing to probing. Changes in AM behavior led to a reduction in the clearance of inhaled bacteria, Pseudomonas aeruginosa. These findings shed light on pathways involved in AM migration and highlight the harmful impact of e-cig vaping on AM function.
-
Immunology and Microbiology
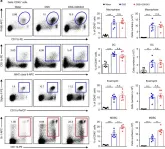
In Nat Commun on 3 June 2019 by Zhou, J., Huang, S., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1