FLT3-L-dependent classical dendritic cells (cDCs) recruit anti-tumor and tumor-protecting lymphocytes. We evaluate cancer growth in mice with low, normal, or high levels of cDCs. Paradoxically, both low or high numbers of cDCs improve survival in mice with melanoma. In low cDC context, tumors are restrained by the adaptive immune system through influx of effector T cells and depletion of Tregs and NK cells. High cDC numbers favor the innate anti-tumor response, with massive recruitment of activated NK cells, despite high Treg infiltration. Anti CTLA-4 but not anti PD-1 therapy synergizes with FLT3-L therapy in the cDCHi but not in the cDCLo context. A combination of cDC boost and Treg depletion dramatically improves survival of tumor-bearing mice. Transcriptomic data confirm the paradoxical effect of cDC levels on survival in several human tumor types. cDCHi-TregLo state in such patients predicts best survival. Modulating cDC numbers via FLT3 signaling may have therapeutic potential in human cancer.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 16
FLT3L-dependent dendritic cells control tumor immunity by modulating Treg and NK cell homeostasis.
In Cell Reports Medicine on 19 December 2023 by Régnier, P., Vétillard, M., et al.
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Cell and Developmental Biology on 31 May 2023 by Ma, S., Li, X., et al.
Neural crest-derived cells play essential roles in skin function and homeostasis. However, how they interact with environmental cues and differentiate into functional skin cells remains unclear. Using a combination of single-cell data analysis, neural crest lineage tracing, and flow cytometry, we found that the expression of integrin α6 (ITGA6) in neural crest and its derivatives was developmentally regulated and that ITGA6 could serve as a functional surface marker for distinguishing neural crest derivatives in the skin. Based on the expression of ITGA6, Wnt1-Cre lineage neural crest derivatives in the skin could be categorized into three subpopulations, namely, ITGA6bright, ITGA6dim, and ITGA6neg, which were found to be Schwann cells, melanocytes, and fibroblasts, respectively. We further analyzed the signature genes and transcription factors that specifically enriched in each cell subpopulation, as well as the ligand or receptor molecules, mediating the potential interaction with other cells of the skin. Additionally, we found that Hmx1 and Lhx8 are specifically expressed in neural crest-derived fibroblasts, while Zic1 and homeobox family genes are expressed in mesoderm-derived fibroblasts, indicating the distinct development pathways of fibroblasts of different origins. Our study provides insights into the regulatory landscape of neural crest cell development and identifies potential markers that facilitate the isolation of different neural crest derivatives in the skin.
Copyright © 2023 Ma, Li, Cao, Zhan, Fu, Xiao and Yang.
-
FC/FACS
-
Mus musculus (House mouse)
Assessing lineage and cytolytic functional potential of murine tissue-resident innate lymphocytes.
In STAR Protocols on 17 March 2023 by Nixon, B. G., Chou, C., et al.
Group 1 innate lymphocytes are heterogeneous, and their ontogeny and function remain ambiguous. Here, we describe a protocol to measure cell ontogeny and effector functions of natural killer (NK) and ILC1 subsets based on current understanding of their differentiation pathways. We use cre drivers to genetically fate-map cells, tracking plasticity between mature NK and ILC1. We describe innate lymphoid cell precursor transfer studies that determine ontogeny of granzyme-C-expressing ILC1. Additionally, we detail in vitro killing assays that test cytolytic potential of ILC1s. For complete details on the use and execution of this protocol, please refer to Nixon et al. (2022).1.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
In European Journal of Immunology on 1 March 2022 by Kragten, N. A. M., Taggenbrock, R. L., et al.
iNKT cells are CD1d-restricted T cells that play a pro-inflammatory or regulatory role in infectious and autoimmune diseases. Thymic precursors of iNKT cells eventually develop into distinct iNKT1, iNKT2, and iNKT17 lineages in the periphery. It remains unclear whether iNKT cells retain developmental potential after lineage commitment. iNKT cells acquire a similar phenotype as tissue-resident memory T cells, suggesting that they also differentiate along a trajectory that enables them to persist in peripheral tissues. Here, we addressed whether lineage commitment and memory differentiation are parallel or sequential developmental programs of iNKT cells. We defined three subsets of peripheral iNKT cells using CD62L and CD69 expression that separate central, effector, and resident memory phenotype cells. The majority of iNKT1 cells displayed a resident phenotype in contrast to iNKT2 and iNKT17 cells. The transcription factor Hobit, which is upregulated in iNKT cells, plays an essential role in their development together with its homolog Blimp-1. Hobit and Blimp-1 instructed the differentiation of central memory iNKT cells into resident memory iNKT cells, but did not impact commitment into iNKT1, iNKT2, or iNKT17 lineages. Thus, we conclude that memory differentiation and the establishment of residency occur after lineage commitment through a Hobit and Blimp-1-driven transcriptional program.
© 2022 Wiley-VCH GmbH.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Journal for Immunotherapy of Cancer on 1 March 2021 by Karaki, S., Blanc, C., et al.
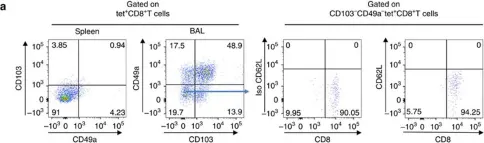
Resident memory T lymphocytes (TRM) are located in tissues and play an important role in immunosurveillance against tumors. The presence of TRM prior to treatment or their induction is associated to the response to anti-Programmed cell death protein 1 (PD-1)/Programmed death-ligand 1 (PD-L1) immunotherapy and the efficacy of cancer vaccines. Previous work by our group and others has shown that the intranasal route of vaccination allows more efficient induction of these cells in head and neck and lung mucosa, resulting in better tumor protection. The mechanisms of in vivo migration of these cells remains largely unknown, apart from the fact that they express the chemokine receptor CXCR6.
We used CXCR6-deficient mice and an intranasal tumor vaccination model targeting the Human Papillomavirus (HPV) E7 protein expressed by the TC-1 lung cancer epithelial cell line. The role of CXCR6 and its ligand, CXCL16, was analyzed using multiparametric cytometric techniques and Luminex assays.Human biopsies obtained from patients with lung cancer were also included in this study.
We showed that CXCR6 was preferentially expressed by CD8+ TRM after vaccination in mice and also on intratumoral CD8+ TRM derived from human lung cancer. We also demonstrate that vaccination of Cxcr6-deficient mice induces a defect in the lung recruitment of antigen-specific CD8+ T cells, preferentially in the TRM subsets. In addition, we found that intranasal vaccination with a cancer vaccine is less effective in these Cxcr6-deficient mice compared with wild-type mice, and this loss of efficacy is associated with decreased recruitment of local antitumor CD8+ TRM. Interestingly, intranasal, but not intramuscular vaccination induced higher and more sustained concentrations of CXCL16, compared with other chemokines, in the bronchoalveolar lavage fluid and pulmonary parenchyma.
This work demonstrates the in vivo role of CXCR6-CXCL16 axis in the migration of CD8+ resident memory T cells in lung mucosa after vaccination, resulting in the control of tumor growth. This work reinforces and explains why the intranasal route of vaccination is the most appropriate strategy for inducing these cells in the head and neck and pulmonary mucosa, which remains a major objective to overcome resistance to anti-PD-1/PD-L1, especially in cold tumors.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
Cancer Research
-
Immunology and Microbiology
In Nat Commun on 24 May 2017 by Nizard, M., Roussel, H., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1