Paroxysmal nocturnal haemoglobinuria (PNH) is a disorder resulting from erythrocyte membrane deficiencies caused by PIG-A gene mutations. While current treatments alleviate symptoms, they fail to address the underlying cause of the disease-the pathogenic PNH clones. In this study, we found that the expression of carbamoyl phosphate synthetase 1 (CPS1) was downregulated in PNH clones, and the level of CPS1 was negatively correlated with the proportion of PNH clones. Using PIG-A knockout K562 (K562 KO) cells, we demonstrated that CPS1 knockdown increased cell proliferation and altered cell metabolism, suggesting that CPS1 participates in PNH clonal proliferation through metabolic reprogramming. Furthermore, we observed an increase in the expression levels of the histone demethylase JMJD1C in PNH clones, and JMJD1C expression was negatively correlated with CPS1 expression. Knocking down JMJD1C in K562 KO cells upregulated CPS1 and H3K36me3 expression, decreased cell proliferation and increased cell apoptosis. Chromatin immunoprecipitation analysis further demonstrated that H3K36me3 regulated CPS1 expression. Finally, we demonstrated that histone demethylase inhibitor JIB-04 can suppressed K562 KO cell proliferation and reduced the proportion of PNH clones in PNH mice. In conclusion, aberrant regulation of the JMJD1C-H3K36me3-CPS1 axis contributes to PNH clonal proliferation. Targeting JMJD1C with a specific inhibitor unveils a potential strategy for treating PNH patients.
© 2024 British Society for Haematology and John Wiley & Sons Ltd.
Product Citations: 26
In British Journal of Haematology on 1 June 2024 by Chen, Y., Liu, H., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cardiovascular biology
-
Cell Biology
-
Genetics
In IScience on 19 April 2024 by Josi, R., Speiser, D. E., et al.
The global incidence of human papillomavirus (HPV) associated head and neck carcinoma is on the rise, in response to this a tetravalent therapeutic vaccine named Qβ-HPVag was developed. This vaccine, utilizing virus-like particles (VLPs) loaded with toll-like receptor ligands and chemically coupled to four HPV16-derived peptides, demonstrated strong anti-tumor effects in a murine head and neck cancer model. Qβ-HPVag impeded tumor progression, increased infiltration of HPV-specific T cells, and significantly improved survival. The vaccine`s efficacy was associated with immune repolarization in the tumor microenvironment, characterized by expanded activated dendritic cell subsets (cDC1, cDC2, DC3). Notably, mice responding to treatment exhibited a higher percentage of migratory DC3 cells expressing CCR7. These findings suggest promising prospects for optimized VLP-based vaccines in treating HPV-associated head and neck cancer.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Nature Communications on 12 April 2024 by Barnkob, M. B., Michaels, Y. S., et al.
Semaphorin-3A (SEMA3A) functions as a chemorepulsive signal during development and can affect T cells by altering their filamentous actin (F-actin) cytoskeleton. The exact extent of these effects on tumour-specific T cells are not completely understood. Here we demonstrate that Neuropilin-1 (NRP1) and Plexin-A1 and Plexin-A4 are upregulated on stimulated CD8+ T cells, allowing tumour-derived SEMA3A to inhibit T cell migration and assembly of the immunological synapse. Deletion of NRP1 in both CD4+ and CD8+ T cells enhance CD8+ T-cell infiltration into tumours and restricted tumour growth in animal models. Conversely, over-expression of SEMA3A inhibit CD8+ T-cell infiltration. We further show that SEMA3A affects CD8+ T cell F-actin, leading to inhibition of immune synapse formation and motility. Examining a clear cell renal cell carcinoma patient cohort, we find that SEMA3A expression is associated with reduced survival, and that T-cells appear trapped in SEMA3A rich regions. Our study establishes SEMA3A as an inhibitor of effector CD8+ T cell tumour infiltration, suggesting that blocking NRP1 could improve T cell function in tumours.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
In British Journal of Pharmacology on 1 September 2023 by Liu, Y., Chen, Y., et al.
MicroRNA-9 (miR-9) has previously been described as a dual-functional RNA during breast cancer progression and its roles need to be clarified thoroughly.
A miR-9 knockout mode of mouse breast cancer, the MMTV-PyMT model (PyMT-miR-9-/- ), combined with different human breast cancer cell lines were used to evaluate the effects of miR-9 on breast cancer initiation, progression and metastasis. Lin-NECs (Neoplastic mammary epithelial cells) and pNECs (Pre-neoplastic mammary epithelial cells) were isolated and subjected to tumour-initiation assay. Whole-mount staining of mammary gland and histology was performed to determine mammary gland growth. Tumour-initiating analysis combining a series of in vitro experiments were carried out to evaluate miR-9 roles in tumour-initiating ability. RNA-sequencing of human breast cancer cells, and mammary glands at hyperplastic stages and established tumours in PyMT and PyMT-miR-9-/- mice, ChIP and luciferase report assays were conducted to reveal the underlying mechanisms.
MiR-9 is ectopically expressed in breast cancer and its level is negatively correlated with the prognosis, especially in basal-like breast cancer patients. Additionally, miR-9 is essential for breast cancer progression by promoting the expansion and activity of tumour-initiating cells (TICs) in preneoplastic glands, established tumours and xenograft modes. Mechanistically, the activity of TICs hinges on a positive TGF-β/miR-9 regulatory loop mediated by the STARD13/YAP axis.
These findings demonstrate that miR-9 is an oncogenic miRNA rather than a tumour-suppressor in breast cancer, calling for rectification of the model for this conserved and highly abundant miRNA.
© 2023 British Pharmacological Society.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Pharmacology
Preprint on BioRxiv : the Preprint Server for Biology on 27 August 2023 by Gurler, S. B., Wagstaff, O., et al.
SUMMARY HER2 is considered as a driver oncogene responsible for the HER2+ subtype of breast cancer. However, it is still unclear how HER2 induces the oncogenic transformation of breast cancer stem cells (BCSCs) and initiates tumorigenesis during premalignant stage breast cancer. Here, we used clinical samples and mouse models of HER2+ breast cancer to demonstrate that neither BCSCs nor their cell-of-origin express HER2/Neu in early-stage breast tumors. Instead, our results demonstrate that Neu overexpression results in the transformation of BCSCs in a non-cell-autonomous manner via triggering DNA damage and somatic mutagenesis in their Neu-negative cell-of-origin. This is caused by the increased oxidative stress in the tissue microenvironment generated by altered energy metabolism and increased reactive oxygen species levels in Neu-overexpressing mammary ducts. Therefore, our findings illustrate a previously unrecognized mechanism of HER2-induced breast tumor initiation in vivo with potential impacts on future preventive treatments for HER2+ premalignant breast cancer.
-
FC/FACS
-
Mus musculus (House mouse)
In Nat Commun on 15 September 2020 by Kim, M. R., Wu, M. J., et al.
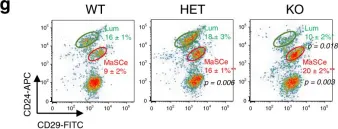
Fig.1.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2
In Nat Commun on 15 September 2020 by Kim, M. R., Wu, M. J., et al.
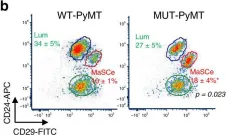
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2