Achieving a cure is an urgent need for patients with advanced solid tumors. Here, we discover that oncolytic virus (OV) infection enhances IL-18 receptor expression but fails to increase IL-18 ligand expression. Therefore, we engineer armed oncolytic alphavirus M1 expressing wild-type IL-18 (wtIL-18) or a mutant variant (mutIL-18) that evades IL-18 binding protein (IL-18BP) while maintaining IL-18 receptor (IL-18R) binding. Intravenous administration of M1-mutIL-18 suppresses the growth of multiple advanced solid tumors in C57BL/6 and BALB/c mouse models and promotes long-term systemic immune memory. Mechanistically, armed M1-mutIL-18 enhances directed clonal expansion and differentiation of CD8+ T cells and sustains IFN-γ production. Thus, armed M1-mutIL-18 promotes dendritic cell (DC) activation, priming and activation of CD8+ T cells in lymphatic organs, and infiltration of IL-18R+ CD8+ T cells in the tumor microenvironment, establishing a positive feedback loop. We further show that a PD-L1 inhibitor enhances the anti-tumor efficacy of mutIL-18 OVs. These results highlight the importance of the IL-18 pathway in oncolytic virus therapy and implicate reprogramming ligand-receptor interaction as an effective strategy for immunotherapy.
© 2025. The Author(s).
Product Citations: 53
In Nature Communications on 3 July 2025 by Fan, Z., Liu, Y., et al.
In Nature Communications on 25 April 2025 by Hu, W., Chen, Z. M., et al.
Helicobacter pylori (H. pylori) manipulates the host immune system to establish a persistent colonization, posing a serious threat to human health, but the mechanisms remain poorly understood. Here we integrate single-cell RNA sequencing and TCR profiling for analyzing 187,192 cells from 11 H. pylori-negative and 12 H. pylori-positive individuals to describe the human gastric ecosystem reprogrammed by H. pylori infection, as manifested by impaired antigen presentation and phagocytosis function. We further delineate a monocyte-to-C1QC+ macrophage differentiation trajectory driven by H. pylori infection, while T cell responses exhibit broad functional impairment and hyporesponsiveness with restricted clonal expansion capacity. We also identify an HLA-DRs- and CTLA4-expressing T cell population residing in H. pylori-inhabited stomach that potentially contribute to immune evasion. Together, our findings provide single-cell resolution information into the immunosuppressive microenvironment shaped by H. pylori infection, offering critical insights for developing novel therapeutic approaches to eliminate this globally prevalent pathogen.
© 2025. The Author(s).
-
Genetics
-
Immunology and Microbiology
In Frontiers in Immunology on 7 April 2025 by Chen, L., Ruan, G., et al.
Immune checkpoint therapy for colorectal cancer (CRC) has been found to be unsatisfactory for clinical treatment. Fecal microbiota transplantation (FMT) has been shown to remodel the intestinal flora, which may improve the therapeutic effect of αPD-1. Further exploration of key genera that can sensitize cells to αPD-1 for CRC treatment and preliminary exploration of immunological mechanisms may provide effective guidance for the clinical treatment of CRC.
In this study, 16S rRNA gene sequencing was analyzed in the fecal flora of both responders and no-responders to αPD-1 treatment, and the therapeutic effect was experimentally verified.
Pseudomonas aeruginosa was found to be highly abundant in the fecal flora of treated mice, and Pseudomonas aeruginosa mannose-sensitive hemagglutinin (PA-MSHA) in combination with αPD-1 was effective in the treatment of CRC through the induction of CD8+ T-cell immunological effects.
The clinical drug PA-MSHA can be used in combination with αPD-1 for the treatment of CRC as a potential clinical therapeutic option.
Copyright © 2025 Chen, Ruan, Zhao, Yi, Xiao, Tian, Cheng, Chen and Wei.
-
Cancer Research
-
Immunology and Microbiology
Targeted labeling and depletion of alveolar macrophages using VeDTR mouse technology.
In IScience on 21 March 2025 by Nakayama, Y., Sasai, M., et al.
Alveolar macrophages (AMs) are essential for maintaining lung homeostasis. However, their roles in respiratory infections have been controversial because the methods of depleting them have often suffered from poor cell selectivity. To resolve this problem, we here used VeDTR technology to generate a transgenic mouse line in which AMs can be specifically depleted using diphtheria toxin. When various respiratory infections were examined using this system, we found that AMs prevented the proliferation of Mycobacterium abscessus. This result differed from previous findings using clodronate liposomes to deplete macrophages. We also revealed that the disappearance of AMs contributes to the reduction of bacterial load in the lungs and that AMs are indispensable for GM-CSF-mediated defense against M. abscessus infection. Taken together, the development of an AM-specific depletion system has provided an opportunity to study the roles of AMs in various respiratory infections from a different perspective.
© 2025 The Author(s).
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 March 2025 by Lepland, A., Peranzoni, E., et al.
In triple-negative breast cancer (TNBC), pro-tumoral macrophages promote metastasis and suppress the immune response. To target these cells, a previously identified CD206 (mannose receptor)-binding peptide, mUNO was engineered to enhance its affinity and proteolytic stability. The new rationally designed peptide, MACTIDE, includes a trypsin inhibitor loop, from the Sunflower Trypsin Inhibitor-I. Binding studies to recombinant CD206 revealed a 15-fold lower KD for MACTIDE compared to parental mUNO. Mass spectrometry further demonstrated a 5-fold increase in MACTIDE's half-life in tumor lysates compared to mUNO. Homing studies in TNBC-bearing mice shows that fluorescein (FAM)-MACTIDE precisely targeted CD206+ tumor-associated macrophages (TAM) upon intravenous, intraperitoneal, and even oral administration, with minimal liver accumulation. MACTIDE was conjugated to Verteporfin, an FDA-approved photosensitizer and YAP/TAZ pathway inhibitor to create the conjugate MACTIDE-V. In the orthotopic 4T1 TNBC mouse model, non-irradiated MACTIDE-V-treated mice exhibited anti-tumoral effects comparable to those treated with irradiated MACTIDE-V, with fewer signs of toxicity, prompting further investigation into the laser-independent activity of the conjugate. In vitro studies using bone marrow-derived mouse macrophages showed that MACTIDE-V excluded YAP from the nucleus, increased phagocytic activity, and upregulated several genes associated with cytotoxic anti-tumoral macrophages. In mouse models of TNBC, MACTIDE-V slowed primary tumor growth, suppressed lung metastases, and increased markers of phagocytosis and antigen presentation in TAM and monocytes, increasing the tumor infiltration of several lymphocyte subsets. MACTIDE-V is proposed as a promising peptide-drug conjugate for modulating macrophage function in breast cancer immunotherapy.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
-
Cancer Research
In Nutrients on 9 September 2020 by Sun, P., Kim, Y., et al.
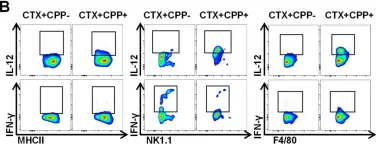
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 2
In Nutrients on 9 September 2020 by Sun, P., Kim, Y., et al.
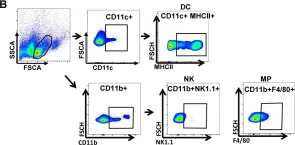
Fig.2.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 2