The microenvironment of hematologic cancers contributes to tumor cell survival and proliferation, as well as treatment resistance. Understanding tumor- and drug-induced changes to the immune cell composition and functionality is therefore critical for implementing optimal treatment strategies and for the development of novel cancer therapies. The liquid nature of peripheral blood makes this organ uniquely suited for single-cell studies by flow cytometry. (Phospho)protein profiles detected by flow cytometry analyses have been shown to correlate with ex vivo drug sensitivity and to predict treatment outcomes in hematologic cancers, demonstrating that this method is suitable for pre-clinical studies. Here, we present a flow cytometry protocol that combines multi-parameter immunophenotyping with single-cell (phospho)protein profiling. The protocol makes use of fluorescent cell barcoding, which means that multiple cell samples, either collected from different donors or exposed to different treatment conditions, can be combined and analyzed as one experiment. This reduces variability between samples, increases the throughput of the experiment, and lowers experimental costs. This protocol may serve as a guide for the use and further development of assays to study immunophenotype and cell signaling at single-cell resolution in normal and malignant cells. The read-outs may provide biological insight into cancer pathogenesis, identify novel drug targets, and ultimately serve as a biomarker to guide clinical decision-making.
© 2024. The Author(s).
Product Citations: 5
In NPJ Precision Oncology on 20 May 2024 by Hermansen, J. U., Yin, Y., et al.
-
Homo sapiens (Human)
RASA2 ablation in T cells boosts antigen sensitivity and long-term function.
In Nature on 1 September 2022 by Carnevale, J., Shifrut, E., et al.
The efficacy of adoptive T cell therapies for cancer treatment can be limited by suppressive signals from both extrinsic factors and intrinsic inhibitory checkpoints1,2. Targeted gene editing has the potential to overcome these limitations and enhance T cell therapeutic function3-10. Here we performed multiple genome-wide CRISPR knock-out screens under different immunosuppressive conditions to identify genes that can be targeted to prevent T cell dysfunction. These screens converged on RASA2, a RAS GTPase-activating protein (RasGAP) that we identify as a signalling checkpoint in human T cells, which is downregulated upon acute T cell receptor stimulation and can increase gradually with chronic antigen exposure. RASA2 ablation enhanced MAPK signalling and chimeric antigen receptor (CAR) T cell cytolytic activity in response to target antigen. Repeated tumour antigen stimulations in vitro revealed that RASA2-deficient T cells show increased activation, cytokine production and metabolic activity compared with control cells, and show a marked advantage in persistent cancer cell killing. RASA2-knockout CAR T cells had a competitive fitness advantage over control cells in the bone marrow in a mouse model of leukaemia. Ablation of RASA2 in multiple preclinical models of T cell receptor and CAR T cell therapies prolonged survival in mice xenografted with either liquid or solid tumours. Together, our findings highlight RASA2 as a promising target to enhance both persistence and effector function in T cell therapies for cancer treatment.
© 2022. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Mcl-1 and Bcl-xL levels predict responsiveness to dual MEK/Bcl-2 inhibition in B-cell malignancies.
In Molecular Oncology on 1 March 2022 by Melvold, K., Giliberto, M., et al.
Most patients with chronic lymphocytic leukemia (CLL) initially respond to targeted therapies, but eventually relapse and develop resistance. Novel treatment strategies are therefore needed to improve patient outcomes. Here, we performed direct drug testing on primary CLL cells and identified synergy between eight different mitogen-activated protein kinase kinase (MEK) inhibitors and the B-cell lymphoma 2 (Bcl-2) antagonist venetoclax. Drug sensitivity was independent of immunoglobulin heavy-chain gene variable region (IGVH) and tumor protein p53 (TP53) mutational status, and CLL cells from idelalisib-resistant patients remained sensitive to the treatment. This suggests that combined MEK/Bcl-2 inhibition may be an option for high-risk CLL. To test whether sensitivity could be detected in other B-cell malignancies, we performed drug testing on cell line models of CLL (n = 4), multiple myeloma (MM; n = 8), and mantle cell lymphoma (MCL; n = 7). Like CLL, MM cells were sensitive to the MEK inhibitor trametinib, and synergy was observed with venetoclax. In contrast, MCL cells were unresponsive to MEK inhibition. To investigate the underlying mechanisms of the disease-specific drug sensitivities, we performed flow cytometry-based high-throughput profiling of 31 signaling proteins and regulators of apoptosis in the 19 cell lines. We found that high expression of the antiapoptotic proteins myeloid cell leukemia-1 (Mcl-1) or B-cell lymphoma-extra large (Bcl-xL) predicted low sensitivity to trametinib + venetoclax. The low sensitivity could be overcome by combined treatment with an Mcl-1 or Bcl-xL inhibitor. Our findings suggest that MEK/Bcl-2 inhibition has therapeutic potential in leukemia and myeloma, and demonstrate that protein expression levels can serve as predictive biomarkers for treatment sensitivities.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
-
Immunology and Microbiology
The receptor for advanced glycation endproducts (RAGE) modulates T cell signaling.
In PLoS ONE on 29 September 2020 by Reed, J. C., Preston-Hurlburt, P., et al.
The receptor for advanced glycation endproducts (RAGE) is expressed in T cells after activation with antigen and is constitutively expressed in T cells from patients at-risk for and with type 1 diabetes mellitus (T1D). RAGE expression was associated with an activated T cell phenotype, leading us to examine whether RAGE is involved in T cell signaling. In primary CD4+ and CD8+ T cells from patients with T1D or healthy control subjects, RAGE- cells showed reduced phosphorylation of Erk. To study T cell receptor signaling in RAGE+ or-T cells, we compared signaling in RAGE+/+ Jurkat cells, Jurkat cells with RAGE eliminated by CRISPR/Cas9, or silenced with siRNA. In RAGE KO Jurkat cells, there was reduced phosphorylation of Zap70, Erk and MEK, but not Lck or CD3ξ. RAGE KO cells produced less IL-2 when activated with anti-CD3 +/- anti-CD28. Stimulation with PMA restored signaling and (with ionomycin) IL-2 production. Silencing RAGE with siRNA also decreased signaling. Our studies show that RAGE expression in human T cells is associated with an activated signaling cascade. These findings suggest a link between inflammatory products that are found in patients with diabetes, other autoimmune diseases, and inflammation that may enhance T cell reactivity.
-
FC/FACS
-
Immunology and Microbiology
In PLoS ONE on 12 September 2015 by George, A. A., Paz, H., et al.
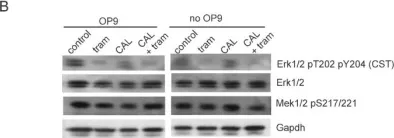
Upstream mutations that lead to constitutive activation of Erk in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) are relatively common. In the era of personalized medicine, flow cytometry could be used as a rapid method for selection of optimal therapies, which may include drugs that target the Erk pathway. Here, we evaluated the utility of phospho-flow, compared to Western blotting, to monitor Erk pathway activation and its inhibition by targeted Mek kinase inhibitors in human BCP ALL. Because the Erk pathway is not only activated endogenously, by mutations, but also by normal extracellular stimulation through stromal contact and serum growth factors, we compared Erk activation ex vivo in ALL cells in the presence and absence of stroma and serum. Phospho-flow was able to readily detect changes in the pool of pErk1/2 that had been generated by normal microenvironmental stimuli in patient-derived BCP-ALL cells passaged in NSG mice, in viably frozen primary patient samples, and in fresh patient samples. Treatment with the Mek1/2 inhibitor selumetinib resulted in a rapid, complete and persistent reduction of microenvironment-generated pErk1/2. Imaging flow cytometry confirmed reduction of nuclear pErk1/2 upon selumetinib treatment. An ALL relapsing with an activating KRasG12V mutation contained higher endogenous as well as serum/stromal-stimulated levels of pErk1/2 than the matched diagnosis sample which lacked the mutation, but selumetinib treatment reduced pErk1/2 to the same level in both samples. Selumetinib and trametinib as Mek inhibitors were mainly cytostatic, but combined treatment with the PI3K∂ inhibitor CAL101 increased cytotoxicity. Thus phospho-flow cytometry could be used as a platform for rapid, individualized in vitro drug sensitivity assessment for leukemia patients at the time of diagnosis.
-
WB
-
Cancer Research
-
Immunology and Microbiology
In PLoS One on 12 September 2015 by George, A. A., Paz, H., et al.
Fig.8.B

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1