Impaired differentiation is a hallmark of myeloid malignancies1,2. Therapies that enable cells to circumvent the differentiation block, such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), are by and large curative in acute promyelocytic leukaemia3, but whether 'differentiation therapy' is a generalizable therapeutic approach for acute myeloid leukaemia (AML) and beyond remains incompletely understood. Here we demonstrate that simultaneous inhibition of the histone demethylase LSD1 (LSD1i) and the WNT pathway antagonist GSK3 kinase4 (GSK3i) robustly promotes therapeutic differentiation of established AML cell lines and primary human AML cells, as well as reducing tumour burden and significantly extending survival in a patient-derived xenograft mouse model. Mechanistically, this combination promotes differentiation by activating genes in the type I interferon pathway via inducing expression of transcription factors such as IRF7 (LSD1i) and the co-activator β-catenin (GSK3i), and their selective co-occupancy at targets such as STAT1, which is necessary for combination-induced differentiation. Combination treatment also suppresses the canonical, pro-oncogenic WNT pathway and cell cycle genes. Analysis of datasets from patients with AML suggests a correlation between the combination-induced transcription signature and better prognosis, highlighting clinical potential of this strategy. Collectively, this combination strategy rewires transcriptional programs to suppress stemness and to promote differentiation, which may have important therapeutic implications for AML and WNT-driven cancers beyond AML.
© 2025. The Author(s).
Product Citations: 11
Perturbing LSD1 and WNT rewires transcription to synergistically induce AML differentiation.
In Nature on 1 June 2025 by Hosseini, A., Dhall, A., et al.
-
Biochemistry and Molecular biology
In International Journal of Molecular Sciences on 18 December 2023 by Mihoub, I., Rharass, T., et al.
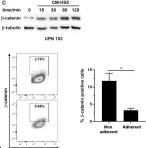
In the microenvironment, cell interactions are established between different cell types to regulate their migration, survival and activation. β-Catenin is a multifunctional protein that stabilizes cell-cell interactions and regulates cell survival through its transcriptional activity. We used chronic lymphocytic leukemia (CLL) cells as a cellular model to study the role of β-catenin in regulating the adhesion of tumor cells to their microenvironment, which is necessary for tumor cell survival and accumulation. When co-cultured with a stromal cell line (HS-5), a fraction of the CLL cells adhere to stromal cells in a dynamic fashion regulated by the different levels of β-catenin expression. In non-adherent cells, β-catenin is stabilized in the cytosol and translocates into the nucleus, increasing the expression of cyclin D1. In adherent cells, the level of cytosolic β-catenin is low but membrane β-catenin helps to stabilize the adhesion of CLL to stromal cells. Indeed, the overexpression of β-catenin enhances the interaction of CLL with HS-5 cells, suggesting that this protein behaves as a regulator of cell adhesion to the stromal component and of the transcriptional regulation of cell survival. Inhibitors that block the stabilization of β-catenin alter this equilibrium and effectively disrupt the support that CLL cells receive from the cross-talk with the stroma.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
In British Journal of Pharmacology on 1 January 2023 by Pagano, E., Romano, B., et al.
Transient receptor potential melastatin type-8 (TRPM8) is a cold-sensitive cation channel protein belonging to the TRP superfamily of ion channels. Here, we reveal the molecular mechanism of TRPM8 and its clinical relevance in colorectal cancer (CRC).
TRPM8 expression and its correlation with the survival rate of CRC patients was analysed. To identify the key pathways and genes related to TRPM8 high expression, Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses were conducted in CRC patients. TRPM8 functional role was assessed by using Trpm8-/- mice in models of sporadic and colitis-associated colon cancer. TRPM8 pharmacological targeting by WS12 was evaluated in murine models of CRC.
TRPM8 is overexpressed in colon primary tumours and in CD326+ tumour cell fraction. TRPM8 high expression was related to lower survival rate of CRC patients, Wnt-Frizzled signalling hyperactivation and adenomatous polyposis coli down-regulation. In sporadic and colitis-associated models of colon cancer, either absence or pharmacological desensitization of TRPM8 reduced tumour development via inhibition of the oncogenic Wnt/β-catenin signalling. TRPM8 pharmacological blockade reduced tumour growth in CRC xenograft mice by reducing the transcription of Wnt signalling regulators and the activation of β-catenin and its target oncogenes such as C-Myc and Cyclin D1.
Human data provide valuable insights to propose TRPM8 as a prognostic marker with a negative predictive value for CRC patient survival. Animal experiments demonstrate TRPM8 involvement in colon cancer pathophysiology and its potential as a drug target for CRC.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
-
IHC-IF
-
Cancer Research
-
Pharmacology
In Cancer Res Commun on 1 September 2022 by Phillips, C., Bhamra, I., et al.
Wnt signaling is implicated in the etiology of gastrointestinal tract cancers. Targeting Wnt signaling is challenging due to on-target toxicity concerns and lack of druggable pathway components. We describe the discovery and characterization of RXC004, a potent and selective inhibitor of the membrane-bound o-acyl transferase Porcupine, essential for Wnt ligand secretion. Absorption, distribution, metabolism, and excretion and safety pharmacology studies were conducted with RXC004 in vitro, and pharmacokinetic exposure assessed in vivo. RXC004 effects on proliferation and tumor metabolism were explored in genetically defined colorectal and pancreatic cancer models in vitro and in vivo. RXC004 effects on immune evasion were assessed in B16F10 immune "cold" and CT26 immune "hot" murine syngeneic models, and in human cell cocultures. RXC004 showed a promising pharmacokinetic profile, inhibited Wnt ligand palmitoylation, secretion, and pathway activation, and demonstrated potent antiproliferative effects in Wnt ligand-dependent (RNF43-mutant or RSPO3-fusion) colorectal and pancreatic cell lines. Reduced tumor growth and increased cancer cell differentiation were observed in SNU-1411 (RSPO3-fusion), AsPC1 and HPAF-II (both RNF43-mutant) xenograft models, with a therapeutic window versus Wnt homeostatic functions. Additional effects of RXC004 on tumor cell metabolism were confirmed in vitro and in vivo by glucose uptake and 18fluorodeoxyglucose-PET, respectively. RXC004 stimulated host tumor immunity; reducing resident myeloid-derived suppressor cells within B16F10 tumors and synergizing with anti-programmed cell death protein-1 (PD-1) to increase CD8+/regulatory T cell ratios within CT26 tumors. Moreover, RXC004 reversed the immunosuppressive effects of HPAF-II cells cocultured with human peripheral blood mononuclear cells, confirming the multiple anticancer mechanisms of this compound, which has progressed into phase II clinical trials.
Wnt pathway dysregulation drives many gastrointestinal cancers; however, there are no approved therapies that target the pathway. RXC004 has demonstrated the potential to block both tumor growth and tumor immune evasion in a genetically defined, clinically actionable subpopulation of Wnt ligand-dependent gastrointestinal cancers. The clinical utility of RXC004, and other Porcupine inhibitors, in such Wnt ligand-dependent cancers is currently being assessed in patient trials.
© 2022 The Authors; Published by the American Association for Cancer Research.
-
Cancer Research
-
Immunology and Microbiology
PXR mediates mifepristone-induced hepatomegaly in mice.
In Acta Pharmacologica Sinica on 1 January 2022 by Yao, X. P., Jiao, T. Y., et al.
Mifepristone (Mif), an effective synthetic steroidal antiprogesterone drug, is widely used for medical abortion and pregnancy prevention. Due to its anti-glucocorticoid effect, high-dose Mif is also used to treat Cushing's syndrome. Mif was reported to active pregnane X receptor (PXR) in vitro and PXR can induce hepatomegaly via activation and interaction with yes-associated protein (YAP) pathway. High-dose Mif was reported to induce hepatomegaly in rats and mice, but the underlying mechanism remains unclear. Here, the role of PXR was studied in Mif-induced hepatomegaly in C57BL/6 mice and Pxr-knockout mice. The results demonstrated that high-dose Mif (100 mg · kg-1 · d-1, i.p.) treatment for 5 days significantly induced hepatomegaly with enlarged hepatocytes and promoted proliferation, but low dose of Mif (5 mg · kg-1 · d-1, i.p.) cannot induce hepatomegaly. The dual-luciferase reporter gene assays showed that Mif can activate human PXR in a concentration-dependent manner. In addition, Mif could promote nuclear translocation of PXR and YAP, and significantly induced the expression of PXR, YAP, and their target proteins such as CYP3A11, CYP2B10, UGT1A1, ANKRD, and CTGF. However, Mif (100 mg · kg-1 · d-1, i.p.) failed to induce hepatomegaly in Pxr-knockout mice, as well as hepatocyte enlargement and proliferation, further indicating that Mif-induced hepatomegaly is PXR-dependent. In summary, this study demonstrated that PXR-mediated Mif-induced hepatomegaly in mice probably via activation of YAP pathway. This study provides new insights in Mif-induced hepatomegaly, and provides novel evidence on the crucial function of PXR in liver enlargement and regeneration.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
-
Pharmacology
In Int J Mol Sci on 18 December 2023 by Mihoub, I., Rharass, T., et al.
Fig.2.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3
In BMC Cancer on 2 August 2013 by Yang, L. H., Han, Y., et al.
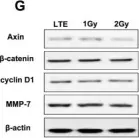
Fig.3.G

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 3
In BMC Cancer on 2 August 2013 by Yang, L. H., Han, Y., et al.
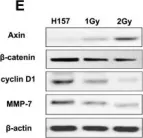
Fig.3.E

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 3