Depletion of circulatory asparagine (Asn) by L-asparaginase (ASNase) has been used for clinical treatment of leukemia, whereas solid tumors are unresponsive to this therapy owing to their active Asn biosynthesis. Herein, we develop a type of core-shell structured cascade-responsive nanoparticles (NPs) for sequential modulation of exogenous Asn supply and endogenous Asn production. The reactive oxygen species-sensitive NP shells disintegrate in the tumor microenvironment and liberate ASNase to scavenge extracellular Asn. The acid-labile NP cores subsequently decompose in the tumor cells and release rotenone to block intracellular Asn biosynthesis. Administration of the dual Asn-depriving NPs in murine models of triple-negative breast cancer and colorectal cancer substantially suppress the growth and epithelial-mesenchymal transition of primary and relapsed tumors, fully eradicate spontaneous and post-surgical metastasis, and confer long-term T cell memory for complete resistance to tumor rechallenge. This study represents a generalized strategy to harness amino acid depletion therapy against solid tumors.
© 2025. The Author(s).
Product Citations: 18
Dual asparagine-depriving nanoparticles against solid tumors.
In Nature Communications on 1 July 2025 by Shen, Y., Wang, H., et al.
-
Cancer Research
In Diabetes Metabolism Journal on 22 May 2025 by Song, D. G., Pak, S., et al.
Obesity is a rapidly increasing global health issue, which is associated with glucose and insulin resistance. Phosphodiesterase type 5 (PDE5) inhibitors (PDE5i) are known for their ability to enhance blood flow and vascular stability and are widely used to treat conditions such as erectile dysfunction, pulmonary hypertension, heart failure, and cancer. However, studies investigating the role of PDE5i in alleviating obesity and metabolic diseases remains unclear. Therefore, we investigated the effects of PDE5i on obesity and metabolic disorders in diet-induced obese mice and its underlying mechanisms.
PDE5i was administered to high-fat diet (HFD)-fed C57BL/6J mice for 6 to 7 weeks. Body weight and food intake were measured weekly, and baseline metabolic rates, physical activity, and glucose and insulin tolerance tests were assessed during PDE5i administration. Macrophages and T-cells in the gonadal white adipose tissue (gWAT) were analyzed by flow cytometry. Vascular stability and blood flow in gWAT were analyzed via immunostaining and in vivo live imaging. RAW264.7 cells and bone marrow-derived macrophages were used to determine immunoregulatory effects of PDE5i.
In HFD-fed mice, PDE5i administration significantly enhanced systemic insulin sensitivity and AKT phosphorylation in gWAT. PDE5i reduced the M1/M2 ratio of gWAT macrophages of obese mice. These phenomena were associated with enhanced blood flow to the gWAT. In vitro experiments revealed that PDE5i suppressed lipopolysaccharide-induced proinflammatory cytokine production and increased the mRNA expression of genes associated with M2 polarization.
PDE5i plays a role in regulating adipose tissue inflammation and thus holds promise as a therapeutic agent for metabolic enhancement.
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Cell Transplantation on 21 February 2025 by Forgioni, A., Watanabe, M., et al.
Pancreatic islet transplantation (PITx) is a promising treatment option for patients with type 1 diabetes mellitus. Previously, we demonstrated that therapy with alloantigen-specific immunomodulatory cells (IMCs) generated ex vivo in the presence of anti-CD80 and CD86 monoclonal antibodies (mAbs), successfully induced tolerance following clinical liver transplantation. To extend IMC therapy to PITx, it is crucial to address the strong inflammatory and innate immune responses that occur immediately after PITx. In this study, we investigated the efficacy of IMCs in modulating macrophage activation and mitigating inflammatory damage of pancreatic islets. IMCs were induced using mouse splenocytes in the presence of anti-mouse anti-CD80 (RM80) and anti-CD86 (GL-1) mAbs. IMCs exerted donor-specific immunosuppressive effects in a mixed lymphocyte reaction. During lipopolysaccharide (LPS) stimulation, the addition of IMCs suppressed conversion to the M1 phenotype and promoted a shift toward the M2 phenotype, particularly under direct cell-cell contact conditions. Nitric oxide production, a hallmark of M1 polarized macrophages, was significantly reduced in LPS-stimulated RAW264 macrophages by IMC treatment. These findings were associated with reduced secretion of pro-inflammatory cytokines, tumoral necrosis factor α, and interleukin-6, and increased interleukin-10 production by macrophages. IMCs effectively prevented macrophage-mediated islet destruction after 12 h of co-culture with LPS-stimulated macrophages and significantly inhibited macrophage migration toward allogeneic islets in vitro. Intraportal co-infusion of IMCs with syngeneic islets in a mouse PITx model resulted in reduced messenger RNA (mRNA) expression of pro-inflammatory cytokines in the recipient liver. Immunohistochemical staining revealed a significantly lower number of F4/80+ macrophages at the transplantation site in IMCs-treated mice. These results demonstrate that IMCs modulate macrophage polarization, promoting a shift toward the M2 phenotype and protecting islets from macrophage-mediated damage. These effects combined with its intrinsic donor antigen-specific immunosuppressive capacity make IMC therapy a promising strategy for improving outcomes after PITx.
-
Immunology and Microbiology
METTL3/miR-192-5p/SCD1 Axis Regulates Lipid Metabolism to Affect T Cell Differentiation in Asthma.
In Mediators of Inflammation on 27 January 2025 by Chen, Z., Yan, D., et al.
Background: This study aimed to explore the mechanisms underlying T-cell differentiation in asthma. Methods and Results: Flow cytometry was performed to detect Th cells. LC-MS/MS was performed to assess lipid metabolism. HE staining was performed to assess the pathological changes of the lung tissues. ELISA was performed to detect cytokine levels. The results of quantitative real-time polymerase chain reaction (qRT-PCR) and western blot showed that miR-192-5p expression was decreased, while SCD1 expression was increased in CD4+T cells isolated from the peripheral blood of children with asthma. The dual luciferase reporter assay determined the direct interaction between miR-192-5p and SCD1. MiR-192-5p inhibitor reduced ASCL3 and PPARα, increased FASN and SREBP1c mRNA expression and protein levels in mouse spleen CD4+T cells, and elevated Th2 and Th17 cells, but these effects were reversed by the SCD1 inhibitor. Oleic acid (OA) reduced Th1 cells and increased Th2 and Th17 cells in mouse spleen CD4+T cells treated with an SCD1 inhibitor. Additionally, pri-miR-192-5p expression was increased in CD4+T cells isolated from the peripheral blood of asthmatic children, and the deletion of METTL3 upregulated pri-miR-192-5p expression in an m6A-dependent manner. MiR-192-5p mimic and inhibitor both reversed miR-192-5p and SCD1 expression affected by overexpression or deletion of METTL3, both in vivo and in vitro. Furthermore, METTL3 overexpression attenuated lung inflammation, elevated Th1 cells, and reduced Th2 and Th17 cells in CD4+T cells isolated from the peripheral blood of asthmatic mice. These effects were reversed by the miR-192-5p inhibitor. Conclusion: These results suggest that METTL3/miR-192-5p/SCD1 axis regulates lipid metabolism and affects T cell differentiation, thus affecting asthma progression. This study may provide novel insights into the pathogenesis of asthma and a new treatment strategy.
Copyright © 2025 Zhengrong Chen et al. Mediators of Inflammation published by John Wiley & Sons Ltd.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
In Journal of Neuroinflammation on 4 October 2024 by Moon, S., Park, Y., et al.
IL-2 regulates T cell differentiation: low-dose IL-2 induces immunoregulatory Treg differentiation, while high-dose IL-2 acts as a potent activator of cytotoxic T cells and NK cells. Therefore, high-dose IL-2 has been studied for use in cancer immunotherapy. We aimed to utilize low-dose IL-2 to treat inflammatory diseases such as obesity and insulin resistance, which involve low-grade chronic inflammation.
Systemic administration of low-dose IL-2 increased Treg cells and decreased inflammation in gonadal white adipose tissue (gWAT), leading to improved insulin sensitivity in high-fat diet-fed obese mice. Additionally, central administration of IL-2 significantly enhanced insulin sensitivity through the activation of the sympathetic nervous system. The sympathetic signaling induced by central IL-2 administration not only decreased interferon γ (IFNγ) + Th1 cells and the expression of pro-inflammatory cytokines, including Il-1β, Il-6, and Il-8, but also increased CD4 + CD25 + FoxP3 + Treg cells and Tgfβ expression in the gWAT of obese mice. These phenomena were accompanied by hypothalamic microgliosis and activation of pro-opiomelanocortin neurons. Furthermore, sympathetic denervation in gWAT reversed the enhanced insulin sensitivity and immune cell polarization induced by central IL-2 administration.
Overall, we demonstrated that IL-2 improves insulin sensitivity through two mechanisms: direct action on CD4 + T cells and via the neuro-immune axis triggered by hypothalamic microgliosis.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
-
Immunology and Microbiology
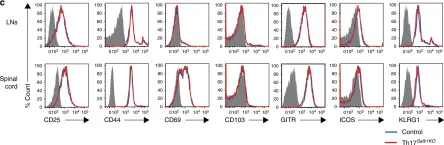
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.3.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1