Metabolic regulators are key in controlling immune cell fate in the tumor microenvironment. The accumulation of tumor-associated macrophages (TAMs) in cancer greatly contributes to metastasis and poor outcome. However, the metabolic pathways responsible for TAM accumulation are largely unknown.
This study aims to elucidate the role of the fatty acid translocase CD36 in the regulation of TAM accumulation.
The immune profile was analyzed in patients with liver metastasis by CIBERSORT. Immunohistostaining of CD68 and CD36 was conducted in clinical specimens from patients with liver metastasis. Myeloid-specific CD36 knockout mice and their littermates were used to establish preclinical liver metastasis models. Subsequently, a series of experiments were used to explore the underlying mechanisms of how CD36 regulates TAM population.
We found that massive TAM accumulation in patients with liver metastasis is associated with an upregulation of CD36 on TAMs. Liver metastasis is abundantly infiltrated by TAMs that are derived from circulating monocytes, but not tissue-resident macrophages. Myeloid-specific CD36 knockout specifically reduced and inactivated monocyte-differentiated macrophages, resulting in diminished immune suppression and attenuated liver metastasis. The protect effects of CD36 knockout can be abrogated by blockade of macrophage recruitment through CCR2 or the p110γ isoform of PI3K downstream of it. Mechanically, CD36 reprogrammed the lipid metabolism of macrophages, in which sphingolipids were significantly downregulated, that contributed to weakened lipid raft-dependent activation of p110γ.
CD36 expands TAM population by promoting the recruitment of circulating monocytes through CCL2/CCR2/p110γ signaling. Our findings provide evidence for targeting CD36 as a therapeutic strategy against liver metastasis.
Copyright © 2024. Published by Elsevier B.V.
Product Citations: 13
In Journal of Advanced Research on 1 August 2025 by Qin, H., Xiao, A., et al.
-
Cancer Research
-
Immunology and Microbiology
Epidermal maintenance of Langerhans cells relies on autophagy-regulated lipid metabolism.
In The Journal of Cell Biology on 3 February 2025 by Arbogast, F., Sal-Carro, R., et al.
Macroautophagy (often-named autophagy), a catabolic process involving autophagy-related (Atg) genes, prevents the accumulation of harmful cytoplasmic components and mobilizes energy reserves in long-lived and self-renewing cells. Autophagy deficiency affects antigen presentation in conventional dendritic cells (DCs) without impacting their survival. However, previous studies did not address epidermal Langerhans cells (LCs). Here, we demonstrate that deletion of either Atg5 or Atg7 in LCs leads to their gradual depletion. ATG5-deficient LCs showed metabolic dysregulation and accumulated neutral lipids. Despite increased mitochondrial respiratory capacity, they were unable to process lipids, eventually leading them to ferroptosis. Finally, metabolically impaired LCs upregulated proinflammatory transcripts and showed decreased expression of neuronal interaction receptors. Altogether, autophagy represents a critical regulator of lipid storage and metabolism in LCs, allowing their maintenance in the epidermis.
© 2024 Arbogast et al.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
Droplet-based proteomics reveals CD36 as a marker for progenitors in mammary basal epithelium.
In Cell Rep Methods on 22 April 2024 by Waas, M., Khoo, A., et al.
Deep proteomic profiling of rare cell populations has been constrained by sample input requirements. Here, we present DROPPS (droplet-based one-pot preparation for proteomic samples), an accessible low-input platform that generates high-fidelity proteomic profiles of 100-2,500 cells. By applying DROPPS within the mammary epithelium, we elucidated the connection between mitochondrial activity and clonogenicity, identifying CD36 as a marker of progenitor capacity in the basal cell compartment. We anticipate that DROPPS will accelerate biology-driven proteomic research for a multitude of rare cell populations.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Droplet based low input proteomic platform for rare cell populations
Preprint on BioRxiv : the Preprint Server for Biology on 11 September 2023 by Waas, M., Khoo, A., et al.
Deep proteomic profiling of rare cell populations has been constrained by sample input requirements. Here, we present DROPPS, an accessible low-input platform that generates high-fidelity proteomic profiles of 100 - 2,500 cells. By applying DROPPS within the mammary epithelium, we elucidated the connection between mitochondrial activity and clonogenicity, discovering and validating CD36 as a marker of progenitor capacity in the basal cell compartment. We anticipate DROPPS will accelerate biology-driven proteomic research for a multitude of rare cell populations.
-
FC/FACS
-
Mus musculus (House mouse)
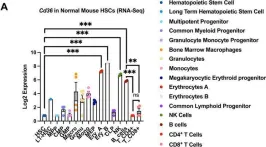
Deletion of CD36 exhibits limited impact on normal hematopoiesis and the leukemia microenvironment.
In Cellular Molecular Biology Letters on 24 May 2023 by Meng, Y., Pospiech, M., et al.
CD36 has been identified as a potential therapeutic target both in leukemic cells and in the tumor immune microenvironment. In acute myeloid leukemia (AML), we found that APOC2 acts with CD36 to promote leukemia growth by activating the LYN-ERK signaling. CD36 also plays a role in lipid metabolism of cancer associated T-cells leading to impaired cytotoxic CD8+ T-cell and enhanced Treg cell function. To establish CD36 as a viable therapeutic target in AML, we investigated whether targeting CD36 has any detrimental impact on normal hematopoietic cells.
Differential expression data of CD36 during human and mouse normal hematopoiesis were examined and compared. Cd36 knockout (Cd36-KO) mice were evaluated for blood analysis, hematopoietic stem cells and progenitors (HSPCs) function and phenotype analyses, and T cells in vitro expansion and phenotypes in comparison with wild type (WT) mice. In addition, MLL-PTD/FLT3-ITD leukemic cells were engrafted into Cd36-KO and WT mice, and leukemia burden was compared between groups.
RNA-Seq data showed that Cd36 expression was low in HSPCs and increased as cells matured. Phenotypic analysis revealed limited changes in blood count except for a slight yet significantly lower red blood cell count and hemoglobin and hematocrit levels in Cd36-KO mice compared with WT mice (P < 0.05). In vitro cell proliferation assays of splenocytes and HSPCs from Cd36-KO mice showed a similar pattern of expansion to that of cells from WT mice. Characterization of HSPCs showed similar percentages of the different progenitor cell populations between Cd36-KO with WT mice. However, Cd36-KO mice exhibited ~ 40% reduction of the number of colonies developed from HSPCs cells compared with WT mice (P < 0.001). Cd36-KO and WT mice presented comparably healthy BM transplant in non-competitive models and developed similar leukemia burden.
Although the loss of Cd36 affects the hematopoietic stem cell and erythropoiesis, limited detrimental overall impact was observed on normal Hematopoietic and leukemic microenvironments. Altogether, considering the limited impact on normal hematopoiesis, therapeutic approaches to target CD36 in cancer are unlikely to result in toxicity to normal blood cells.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
In Cell Mol Biol Lett on 24 May 2023 by Meng, Y., Pospiech, M., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Cell Mol Biol Lett by CiteAb, provided under a CC-BY license
Image 1 of 3
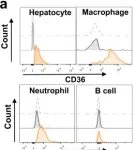
In Nat Commun on 2 October 2022 by Yang, P., Qin, H., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 3
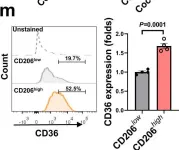
In Nat Commun on 2 October 2022 by Yang, P., Qin, H., et al.
Fig.3.M

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 3