Limited activation and infiltration of CD8+ T cells are major challenges facing T cell-based immunotherapy for most solid tumors, of which the mechanism is multilayered and not yet fully understood.
Levels of CD93 expression on monocytes from paired non-tumor, peritumor and tumor tissues of human hepatocellular carcinoma (HCC) were evaluated. The underlying mechanisms mediating effects of CD93+ monocytes on the inhibition and tumor exclusion of CD8+ T cells were studied through both in vitro and in vivo experiments.
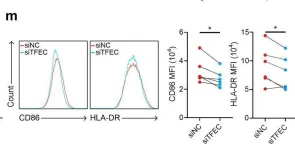
In this study, we found that monocytes in the peritumoral tissues of HCC significantly increased levels of CD93 expression, and these CD93+ monocytes collocated with CD8+ T cells, whose density was much higher in peritumor than intratumor areas. In vitro experiments showed that glycolytic switch mediated tumor-induced CD93 upregulation in monocytes via the Erk signaling pathway. CD93 on the one hand could enhance PD-L1 expression through the AKT-GSK3β axis, while on the other hand inducing monocytes to produce versican, a type of matrix component which interacted with hyaluronan and collagens to inhibit CD8+ T cell migration. Consistently, levels of CD93+ monocytes positively correlated with the density of peritumoral CD8+ T cells while negatively correlated with that of intratumoral CD8+ T cells. Targeting CD93 on monocytes not only increased the infiltration and activation of CD8+ T cells but also enhanced tumor sensitivity to anti-PD-1 treatment in mice in vivo.
This study identified an important mechanism contributing to the activation and limited infiltration of CD8+ T cells in solid tumors, and CD93+ monocytes might represent a plausible immunotherapeutic target for the treatment of HCC.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Product Citations: 14
In Journal for Immunotherapy of Cancer on 23 October 2024 by Jiang, D., Huang, A., et al.
-
FC/FACS
-
Immunology and Microbiology
Cervical mucosal inflammation expands functional polymorphonuclear myeloid-derived suppressor cells
Preprint on MedRxiv : the Preprint Server for Health Sciences on 10 July 2024 by Pieren, D. K., Benítez-Martínez, A., et al.
The mucosal immune system plays a fundamental role in maintaining microbial balance. Microbial imbalance in the female genital tract increases the risk for adverse health outcomes in women and may increase susceptibility to genital tract infections. Among different relevant immune subsets, myeloid-derived suppressor cells (MDSCs) remain understudied in the context of female genital tract conditions. Here we show that frequency of polymorphonuclear (PMN-) MDSCs increased in the cervical mucosa of women with Chlamydia trachomatis , bacterial vaginosis, or with a coinfection, but not in women with human papillomavirus. Mucosal PMN-MDSC frequencies correlated with mucosal IL-1β in C. trachomatis patients and ex vivo exposure of cervical tissue to C. trachomatis elevated both PMN-MDSC frequencies and IL-1β secretion. Likewise, exposure of cervical tissue to cervicovaginal lavage fluid from C. trachomatis and bacterial vaginosis patients also enhanced PMN-MDSC frequencies. Lastly, cervical MDSCs expressed suppressive mediators and functionally suppressed cytotoxic T-cell responses. Our study identifies IL-1β-stimulated PMN-MDSCs as an immune suppressive mediator in female genital tract infections, potentially contributing to susceptibility to acquiring secondary infections at this site.
-
Immunology and Microbiology
In Frontiers in Immunology on 9 May 2024 by Pavlidis, M. A., Viborg, N., et al.
Pharmacodynamic assessment of T-cell-based cancer immunotherapies often focus on detecting rare circulating T-cell populations. The therapy-induced immune cells in blood-derived clinical samples are often present in very low frequencies and with the currently available T-cell analytical assays, amplification of the cells of interest prior to analysis is often required. Current approaches aiming to enrich antigen-specific T cells from human Peripheral Blood Mononuclear Cells (PBMCs) depend on in vitro culturing in presence of their cognate peptides and cytokines. In the present work, we improved a standard, publicly available protocol for T-cell immune analyses based on the in vitro expansion of T cells. We used PBMCs from healthy subjects and well-described viral antigens as a model system for optimizing the experimental procedures and conditions. Using the standard protocol, we first demonstrated significant enrichment of antigen-specific T cells, even when their starting frequency ex vivo was low. Importantly, this amplification occurred with high specificity, with no or neglectable enrichment of irrelevant T-cell clones being observed in the cultures. Testing of modified culturing timelines suggested that the protocol can be adjusted accordingly to allow for greater cell yield with strong preservation of the functionality of antigen-specific T cells. Overall, our work has led to the refinement of a standard protocol for in vitro stimulation of antigen-specific T cells and highlighted its reliability and reproducibility. We envision that the optimized protocol could be applied for longitudinal monitoring of rare blood-circulating T cells in scenarios with limited sample material.
Copyright © 2024 Pavlidis, Viborg, Lausen, Rønø and Kleine-Kohlbrecher.
-
Homo sapiens (Human)
-
Immunology and Microbiology
The immune-suppressive landscape in lepromatous leprosy revealed by single-cell RNA sequencing.
In Cell Discovery on 11 January 2022 by Mi, Z., Wang, Z., et al.
Lepromatous leprosy (L-LEP), caused by the massive proliferation of Mycobacterium leprae primarily in macrophages, is an ideal disease model for investigating the molecular mechanism of intracellular bacteria evading or modulating host immune response. Here, we performed single-cell RNA sequencing of both skin biopsies and peripheral blood mononuclear cells (PBMCs) of L-LEP patients and healthy controls. In L-LEP lesions, we revealed remarkable upregulation of APOE expression that showed a negative correlation with the major histocompatibility complex II gene HLA-DQB2 and MIF, which encodes a pro-inflammatory and anti-microbial cytokine, in the subset of macrophages exhibiting a high expression level of LIPA. The exhaustion of CD8+ T cells featured by the high expression of TIGIT and LAG3 in L-LEP lesions was demonstrated. Moreover, remarkable enhancement of inhibitory immune receptors mediated crosstalk between skin immune cells was observed in L-LEP lesions. For PBMCs, a high expression level of APOE in the HLA-DRhighFBP1high monocyte subset and the expansion of regulatory T cells were found to be associated with L-LEP. These findings revealed the primary suppressive landscape in the L-LEP patients, providing potential targets for the intervention of intracellular bacteria caused persistent infections.
© 2021. The Author(s).
-
Genetics
-
Immunology and Microbiology
In Thoracic Cancer on 1 October 2021 by Yin, W., Lv, J., et al.
Immune cells and molecules are considered as clinical biomarkers and potential targets for immunotherapy. Analyses of the composition of peripheral blood cells hold promise for providing a basis for diagnosing and prognosis lung cancer. In this study, we assessed correlations between immune cell subset profiles in peripheral blood and disease prognosis in patients with lung cancer.
One hundred and thirteen patients with lung cancer and 99 age-matched healthy people were enrolled in this study. The percentage and cell count of monocytes, neutrophils, T cells, B cells, natural killer (NK), and NKT cells in peripheral blood were analyzed by flow cytometry or peripheral blood analyzer. Serum cytokines and colony-stimulating factors were detected by enzyme-linked immunosorbent assay (ELISA).
A reduction in antitumor NK cells (p < 0.0001) and an increase in the protumor MDSCs (p < 0.0001) were observed in the lung cancer patients compared with the controls. Monocyte counts were significantly higher in lung cancer patients with histories of smoking (p < 0.05) or drinking (p < 0.01) than in patients with no relevant history or healthy controls. The number of neutrophils and the neutrophil-to-lymphocyte ratio (NLR) were particularly higher in patients with liver metastasis (p < 0.01) compared with no metastasis patients or healthy controls. Levels of the monocyte-derived cytokine interleukin-6 (p < 0.05), granulocyte colony-stimulating factor (G-CSF) (p < 0.0001), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (p < 0.0001) were higher in patients than in controls. G-CSF levels decreased during the remission phase (p < 0.05), and positively correlated with carbohydrate antigen 19-9 (p < 0.05) and gene mutation (p < 0.05).
Monocyte and neutrophil counts were higher in peripheral blood in lung cancer patients than in controls, especially when patients had histories of smoking, drinking, and liver metastasis. Serum levels of G-CSF and GM-CSF were higher in lung cancer patients, and G-CSF levels positively correlated with disease severity.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
-
FC/FACS
-
Cancer Research
-
Cardiovascular biology
In Cell Res on 1 November 2020 by Chen, Y. P., Yin, J. H., et al.
Fig.3.M

-
FC/FACS
-
Collected and cropped from Cell Res by CiteAb, provided under a CC-BY license
Image 1 of 1