Human pluripotent stem cells (hPSCs) represent a powerful model system to study early developmental processes. However, lineage specification into trophectoderm (TE) and trophoblast (TB) differentiation remains poorly understood, and access to well-characterized placental cells for biomedical research is limited, largely depending on fetal tissues or cancer cell lines. Here, we developed novel strategies enabling highly efficient TE specification that generates cytotrophoblast (CTB) and multinucleated syncytiotrophoblast (STB), followed by the establishment of trophoblast stem cells (TSCs) capable of differentiating into extravillous trophoblast (EVT) and STB after long-term expansion. We confirmed stepwise and controlled induction of lineage- and cell-type-specific genes consistent with developmental biology principles and benchmarked typical features of placental cells using morphological, biochemical, genomics, epigenomics, and single-cell analyses. Charting a well-defined roadmap from hPSCs to distinct placental phenotypes provides invaluable opportunities for studying early human development, infertility, and pregnancy-associated diseases.
© 2024 The Author(s).
Product Citations: 13
Highly efficient generation of self-renewing trophoblast from human pluripotent stem cells.
In IScience on 18 October 2024 by Slamecka, J., Ryu, S., et al.
-
Stem Cells and Developmental Biology
In Frontiers in Oncology on 27 December 2022 by Dardare, J., Witz, A., et al.
Damage specific DNA binding protein 2 (DDB2) is an UV-indiced DNA damage recognition factor and regulator of cancer development and progression. DDB2 has dual roles in several cancers, either as an oncogene or as a tumor suppressor gene, depending on cancer localization. Here, we investigated the unresolved role of DDB2 in pancreatic ductal adenocarcinoma (PDAC).
The expression level of DDB2 in pancreatic cancer tissues and its correlation with patient survival were evaluated using publicly available data. Two PDAC cell models with CRISPR-modified DDB2 expression were developed: DDB2 was repressed in DDB2-high T3M4 cells (T3M4 DDB2-low) while DDB2 was overexpressed in DDB2-low Capan-2 cells (Capan-2 DDB2-high). Immunofluorescence and qPCR assays were used to investigate epithelial-to-mesenchymal transition (EMT) in these models. Migration and invasion properties of the cells were also determined using wound healing and transwell assays. Sensitivity to 5-fluorouracil (5-FU), oxaliplatin, irinotecan and gemcitabine were finally investigated by crystal violet assays.
DDB2 expression level was reduced in PDAC tissues compared to normal ones and DDB2-low levels were correlated to shorter disease-free survival in PDAC patients. DDB2 overexpression increased expression of E-cadherin epithelial marker, and decreased levels of N-cadherin mesenchymal marker. Conversely, we observed opposite effects in DDB2 repression and enhanced transcription of SNAIL, ZEB1, and TWIST EMT transcription factors (EMT-TFs). Study of migration and invasion revealed that these properties were negatively correlated with DDB2 expression in both cell models. DDB2 overexpression sensitized cells to 5-fluorouracil, oxaliplatin and gemcitabine.
Our study highlights the potential tumor suppressive effects of DDB2 on PDAC progression. DDB2 could thus represent a promising therapeutic target or biomarker for defining prognosis and predicting chemotherapy response in patients with PDAC.
Copyright © 2022 Dardare, Witz, Betz, Francois, Meras, Lamy, Lambert, Grandemange, Husson, Rouyer, Demange, Merlin, Harlé and Gilson.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
Preprint on Research Square on 7 April 2022 by Zhang, J., Deng, Y., et al.
h4>Purpose: /h4> This study aimed to investigate the effect of transforming growth factor-β1 (TGF-β1) on the migration capacity of rat vascular smooth muscle cells (VSMCs) and its potential mechanism. h4>Methods: /h4>: Different concentrations of TGF-β1 were used to treat rat VSMCs. The migration capacity of cells was observed by wound healing assay and Transwell assay. Western Blotting and Reverse transcription-quantitative real-time polymerase chain reaction (RT-qPCR) were performed to examine the expression of EMT related gene. h4>Results: /h4> In a certain concentration range, the migration ability of rat VSMCs was enhanced with the increase of TGF-β1 concentration. Meanwhile, TGF-β1 increased the expression of N-cadherin and Vimentin (mesenchymal markers), and decreased the expression of E-cadherin and β-catenin (epithelial markers) at both protein and mRNA levels. h4>Conclusion: /h4> TGF-β1 promoted abnormal migration of rat VSMCs by regulating the expression of EMT related genes. This may be one of the mechanisms of in-stent restenosis (ISR) in coronary arteries.
In Neoplasma on 1 January 2022 by Wu, K. Y., Xie, H., et al.
Emerin (EMD) plays diverse roles in cellular polarity organization, nuclear stability, and cell motility, however, the biological role of EMD relevant to the migration and invasion of hepatocellular carcinoma (HCC) cells has not yet been illustrated. In the present study, we initially found that the upregulation of EMD in HCC tissues, and EMD expression was negatively correlated with the spontaneous metastatic potential of HCC cell lines. Loss of EMD in HCC cells facilitated cell migration and invasion in vitro and metastasis in vivo. Meanwhile, we demonstrated that EMD knockdown induced EMT but enhanced p21 expression in HCC cells. Notably, silencing of EMD in HCC cells increased the cytoplasmic localization of p21 protein, whereas p21 knockdown partially abrogated the migratory and invasive ability, EMT, and the actin cytoskeleton rearrangement induced by EMD knockdown in HCC cells. Our results indicated a significant role of EMD knockdown in the HCC cell motility and metastasis through upregulating the cytoplasmic p21, unveiling a novel mechanism of cell motility regulation induced by EMD.
-
Cancer Research
-
Cell Biology
Overexpression of the nucleoporin Nup88 stimulates migration and invasion of HeLa cells.
In Histochemistry and Cell Biology on 1 November 2021 by Makise, M., Uchimura, R., et al.
Elevated expression of the nucleoporin Nup88, a constituent of the nuclear pore complex, is seen in various types of malignant tumors, but whether this overexpression contributes to the malignant phenotype has yet to be determined. Here, we investigated the effect of the overexpression of Nup88 on the migration and invasion of cervical cancer HeLa cells. The overexpression of Nup88 promoted a slight but significant increase in both migration and invasion, whereas knockdown of Nup88 by RNA interference suppressed these phenotypes. The observed phenotypes in Nup88-overexpressing HeLa cells were not due to the progression of the epithelial-to-mesenchymal transition or activation of NF-κB, which are known to be important for cell migration and invasion. Instead, we identified an upregulation of matrix metalloproteinase-12 (MMP-12) at both the gene and protein levels in Nup88-overexpressing HeLa cells. Upregulation of MMP-12 protein by the overexpression of Nup88 was also observed in one other cervical cancer cell line and two prostate cancer cell lines but not 293 cells. Treatment with a selective inhibitor against MMP-12 enzymatic activity significantly suppressed the invasive ability of HeLa cells induced by Nup88 overexpression. Taken together, our results suggest that overexpression of Nup88 can stimulate malignant phenotypes including invasive ability, which is promoted by MMP-12 expression.
© 2021. The Author(s).
-
Cell Biology
In Cell Death Dis on 7 May 2020 by Chang, T. H., Shanti, R. M., et al.
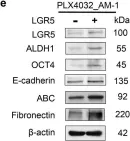
Fig.6.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 2
In Cell Death Dis on 7 May 2020 by Chang, T. H., Shanti, R. M., et al.
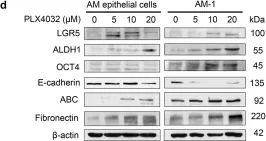
Fig.6.D

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 2