Myocarditis is a life-threatening inflammatory disease, but lacks effective treatment options. Hydroxychloroquine (HCQ), an established antimalarial agent, is used widely to manage rheumatic disorders. This research aimed to evaluate the efficacy of HCQ in treating myocarditis.
A mouse model of experimental autoimmune myocarditis (EAM) was used to evaluate the therapeutic effects of HCQ on cardiac function, inflammation and fibrosis. Echocardiography, histology and cytokine assays were performed to assess cardiac function and inflammatory responses. Single-cell RNA sequencing was employed to analyse immune cell populations and chemotactic activity. C-X-C motif chemokine ligand 16 (CXCL16) levels were measured in cardiac tissue and serum, while YY1 expression was measured by western blotting in macrophages and cardiac tissue. Flow cytometry was used to evaluate immune cell infiltration and migration.
HCQ improved cardiac function in acute and chronic myocarditis. HCQ treatment reduced inflammation, fibrosis and immune cell infiltration in myocarditis models. Single-cell RNA sequencing revealed that HCQ lowered inflammatory cell proportions and suppressed macrophage chemotaxis. HCQ reduced YY1 levels, leading to the down-regulation of CXCL16 expression in macrophages and inhibition of CXCL16-mediated chemotaxis to Th17 and natural killer T (NKT) cells. CXCL16 neutralizing antibodies improved cardiac function and reduced inflammation in myocarditis.
HCQ improves cardiac function and reduces inflammation in myocarditis by inhibiting CXCL16 expression in macrophages, by suppressing its transcription factor YY1, which in turn reduced the chemotaxis of Th17 and NKT cells. HCQ is a promising therapeutic agent for myocarditis.
© 2025 British Pharmacological Society.
Product Citations: 31
In British Journal of Pharmacology on 1 August 2025 by Xuan, Y., Gao, X., et al.
-
Immunology and Microbiology
-
Pharmacology
In PLoS Pathogens on 1 January 2025 by Shen, Z., Li, C., et al.
Vaccines are widely regarded as one of the most effective strategies for combating infectious diseases. However, significant challenges remain, such as insufficient antibody levels, limited protection against rapidly evolving variants, and poor immune durability, particularly in subunit vaccines, likely due to their short in vivo exposure. Recent advances in extending the half-life of protein therapeutics have shown promise in improving drug efficacy, yet whether increasing in vivo persistence can enhance the efficacy of subunit vaccines remains underexplored. In this study, we developed two trimeric SARS-CoV-2 subunit vaccines with distinct pharmacokinetic profiles to evaluate the impact of vaccine persistence on immune efficacy. A self-assembling trimeric subunit vaccine (RBD-HR/trimer) was designed, followed by an extended-persistence variant (RBD-sFc-HR/trimer) incorporating a soluble monomeric IgG1 fragment crystallizable. We demonstrated that RBD-sFc-HR/trimer elicited more robust and higher levels of neutralizing antibodies, with potent and broad neutralization activity against multiple SARS-CoV-2 variants. Notably, RBD-sFc-HR/trimer induced a durable immune response, significantly increasing the number of memory B cells and T cells. This study provides critical insights for designing vaccines that achieve potent and long-lasting immune responses against infectious diseases.
Copyright: © 2025 Shen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Maternal immune activation does not affect maternal microchimeric cells.
In Biology Open on 15 December 2024 by Borges, A. & Irie, N.
We are naturally chimeras. Apart from our own cells originating from the fertilized egg, placental mammals receive small numbers of maternal cells called maternal microchimerism (MMc) that persist throughout one's whole life. Not only are varying frequencies of MMc cells reported in seemingly contradicting phenomena, including immune tolerance and possible contribution to autoimmune-like disease, but frequencies are observable even among healthy littermates showing varying MMc frequencies and cell type repertoire. These varying differences in MMc frequencies or cell types could be contributing to the diverse phenomena related to MMc. However, factors biasing these MMc differences remain largely unknown. Here, we tested whether immunological activation leads to differing MMc frequencies, based on our recent study that suggests that most maternal cells are immune-related. Unexpectedly, fluorescence-activated cell sorting analysis on the murine spleen, thymus, and liver following maternal immune activation by mid-gestational lipopolysaccharide intraperitoneal injections detected no significant difference in the number, or ratio of, immune-related maternal cells in the tested embryonic organs of healthy offspring. These findings suggest that MMc frequencies remain stable even under immune-activated conditions, implying a possible control system of MMc migration against changes in the immunological conditions.
© 2024. Published by The Company of Biologists.
-
Immunology and Microbiology
Combinatorial leaky probiotic for anticancer immunopotentiation and tumor eradication.
In Cell Reports Medicine on 19 November 2024 by Liu, C. H., Pan, Y. C., et al.
Combination therapies present a compelling therapeutic regimen against the immunosuppressive and heterogeneous microenvironment of solid tumors. However, incorporating separate therapeutic modalities in regimen designs can be encumbered by complex logistical, manufacturing, and pharmacokinetic considerations. Herein, we demonstrate a single-vector combinational anticancer therapy using an lpp gene knockout leaky probiotic for simultaneous secretion of immunotherapeutic and oncolytic effector molecules. Through fusion protein design and vector optimization, a Nissle1917 (EcN) bacteria vector is engineered to secrete Neoleukin-2/15 (Neo-2/15) cytokine-functionalized anti-PDL1 nanobody (aPDL1-Neo2/15) and anti-mesothelin-functionalized hemolysin E (HlyE-aMSLN). The multifunctional leaky probiotic enables synchronous immune activation and tumor-targeted cytolytic activity for effective tumor suppression, elevation of tumor immune cell infiltration, and establishment of anticancer immunological memory. lpp gene knockout is further shown to improve probiotic tolerability and intravenous applicability, offering a therapeutically viable approach for combination regimen development.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Cancer Research
In Cell Reports Medicine on 15 October 2024 by Wu, J., Bloch, N., et al.
The clinical use of interleukin-2 (IL-2) for cancer immunotherapy is limited by severe toxicity. Emerging IL-2 therapies with reduced IL-2 receptor alpha (IL-2Rα) binding aim to mitigate toxicity and regulatory T cell (Treg) expansion but have had limited clinical success. Here, we show that IL-2Rα engagement is critical for the anti-tumor activity of systemic IL-2 therapy. A "non-α" IL-2 mutein induces systemic expansion of CD8+ T cells and natural killer (NK) cells over Tregs but exhibits limited anti-tumor efficacy. We develop a programmed cell death protein 1 (PD-1)-targeted, receptor-masked IL-2 immunocytokine, PD1-IL2Ra-IL2, which attenuates systemic IL-2 activity while maintaining the capacity to engage IL-2Rα on PD-1+ T cells. Mice treated with PD1-IL2Ra-IL2 show no systemic toxicities observed with unmasked IL-2 treatment yet achieve robust tumor growth control. Furthermore, PD1-IL2Ra-IL2 can be effectively combined with other T cell-mediated immunotherapies to enhance anti-tumor responses. These findings highlight the therapeutic potential of PD1-IL2Ra-IL2 as a targeted, receptor-masked, and "α-maintained" IL-2 therapy for cancer.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Cancer Research
-
Immunology and Microbiology
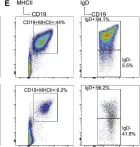
In Mol Cell on 17 May 2018 by Bellelli, R., Borel, V., et al.
Fig.2.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Mol Cell by CiteAb, provided under a CC-BY license
Image 1 of 1