Current antiretroviral therapy (ART) for HIV infection reduces plasma viral loads to undetectable levels and has increased the life expectancy of people with HIV (PWH). However, this increased lifespan is accompanied by signs of accelerated aging and a higher prevalence of age-related comorbidities. Tat (Trans-Activator of Transcription) is a key protein for viral replication and pathogenesis. Tat is encoded by 2 exons, with the full-length Tat ranging from 86 to 101 aa (Tat101). Introducing a stop codon in position 73 generates a 1 exon, synthetic 72aa Tat (Tat72). Intracellular, full-length Tat activates the NF-κB pro-inflammatory pathway and increases antiapoptotic signals and ROS generation. These effects may initiate a cellular senescence program, characterized by cell cycle arrest, altered cell metabolism, and increased senescence-associated secretory phenotype (SASP) mediator release However, the precise role of HIV-Tat in inducing a cellular senescence program in CD4+ T-cells is currently unknown.
Jurkat Tetoff cell lines stably transfected with Tat72, Tat101, or an empty vector were used. Flow cytometry and RT-qPCR were used to address senescence biomarkers, and 105 mediators were assessed in cell supernatants with an antibody-based membrane array. Key results obtained in Jurkat-Tat cells were addressed in primary, resting CD4+ T-cells by transient electroporation of HIV-Tat-FLAG plasmid DNA.
In the Jurkat cell model, expression of Tat101 increased the levels of the senescence biomarkers BCL-2, CD87, and p21, and increased the release of sCD30, PDGF-AA, and sCD31, among other factors. Tat101 upregulated CD30 and CD31 co-expression in the Jurkat cell surface, distinguishing these cells from Tat72 and Tetoff Jurkats. The percentage of p21+, p16+, and γ-H2AX+ cells were higher in Tat-expressing CD4+ T-cells, detected as a FLAG+ population compared to their FLAG- (Tat negative) counterparts. Increased levels of sCD31 and sCD26 were also detected in electroporated CD4+ T-cell supernatants.
Intracellular, full-length HIV-Tat expression increases several senescence biomarkers in Jurkat and CD4+ T-cells, and SASP/Aging mediators in cell supernatants. Intracellular HIV-Tat may initiate a cellular senescence program, contributing to the premature aging phenotype observed in PWH.
Copyright © 2025 Casanova, Rodríguez-Agustín, Ayala-Suárez, Moraga, Maleno, Mallolas, Martínez, Sánchez-Palomino, Miró, Alcamí and Climent.
Product Citations: 54
HIV-Tat upregulates the expression of senescence biomarkers in CD4+ T-cells.
In Frontiers in Immunology on 9 May 2025 by Casanova, V., Rodríguez-Agustín, A., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
A Checkpoint Reversal Receptor Mediates Bipartite Activation and Enhances CAR T-cell Function.
In Cancer Res Commun on 1 March 2025 by Landi, D., Navai, S. A., et al.
The efficacy of chimeric antigen receptor T cells (CART) in solid tumors is limited by immune inhibition. In our study, we observed that effector cytokines mediated the upregulation of the PD-L1 immune checkpoint in primary glioblastoma. To offset the PD-L1 inhibitory signal, we engineered PD-1 checkpoint reversal receptors (CPR) with a CD28 or 41BB costimulatory endodomain and coexpressed them with a first-generation or a CD28-containing second-generation HER2-specific CAR (CPR/CART) using bicistronic vectors. We found that bipartite T-cell activation, by CAR-generated signal 1 and CPR costimulation (signal 2), fine-tuned proinflammatory cytokine release and sustained antitumor activity. Whereas both CPR28 and CPR41BB effectively counteracted the PD-1 signal in vitro, CPR41BB, when coexpressed with a first-generation CAR (CARζ/CPR41BB), promoted central memory differentiation following repeat antigenic stimulation. CARζ/CPR41BB T cells formed a robust immune synapse with tumor targets, similar to a 41BB-containing second-generation CART, maintained the favorable metabolic parameters associated with 41BB costimulation, and demonstrated superior antitumor function after adoptive transfer in xenograft models of gioblastoma and metastatic osteosarcoma. Thus, a CPR molecule with 41BB costimulation that curtails PD-1 inhibition and complements CAR signaling to optimize T-cell activation could enhance CART efficacy against solid tumors.
Enhancing CART function and persistence while balancing immune effector-mediated inflammation is crucial. Using our clinically relevant HER2-CAR platform, we demonstrate that tumor-intrinsic signals like the PD-1/PD-L1 immune checkpoint can be leveraged in CART design to modulate immune synapse and metabolic parameters, improving antitumor function without increasing cytokine production.
©2025 The Authors; Published by the American Association for Cancer Research.
-
Immunology and Microbiology
The antitumor activity of TGFβ-specific T cells is dependent on IL-6 signaling.
In Cellular Molecular Immunology on 1 January 2025 by Perez-Penco, M., Byrdal, M., et al.
Although interleukin (IL)-6 is considered immunosuppressive and tumor-promoting, emerging evidence suggests that it may support antitumor immunity. While combining immune checkpoint inhibitors (ICIs) and radiotherapy in patients with pancreatic cancer (PC) has yielded promising clinical results, the addition of an anti-IL-6 receptor (IL-6R) antibody has failed to elicit clinical benefits. Notably, a robust TGFβ-specific immune response at baseline in PC patients treated solely with ICIs and radiotherapy correlated with improved survival. Recent preclinical studies demonstrated the efficacy of a TGFβ-based immune modulatory vaccine in controlling PC tumor growth, underscoring the important role of TGFβ-specific immunity in PC. Here, we explored the importance of IL-6 for TGFβ-specific immunity in PC. In a murine model of PC, coadministration of the TGFβ-based immune modulatory vaccine with an anti-IL-6R antibody rendered the vaccine ineffective. IL-6R blockade hampered the development of vaccine-induced T-cells and tumoral T-cell infiltration. Furthermore, it impaired the myeloid population, resulting in increased tumor-associated macrophage infiltration and an enhanced immunosuppressive phenotype. In PC patients, in contrast to those receiving only ICIs and radiotherapy, robust TGFβ-specific T-cell responses at baseline did not correlate with improved survival in patients receiving ICIs, radiotherapy and IL-6R blockade. Peripheral blood immunophenotyping revealed that IL-6R blockade altered the T-cell and monocytic compartments, which was consistent with the findings in the murine model. Our data suggest that the antitumor efficacy of TGFβ-specific T cells in PC depends on the presence of IL-6 within the tumor. Consequently, caution should be exercised when employing IL-6R blockade in patients receiving cancer immunotherapy.
© 2024. The Author(s).
-
Immunology and Microbiology
Temperature-based MHC class-I multimer peptide exchange for human HLA-A, B and C
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2024 by Pothast, C. R., Derksen, I., et al.
T cell recognition of specific antigens presented by major histocompatibility complexes class-I (MHC-I) can play an important role during immune responses against pathogens and cancer cells. Detection of T cell immunity is based on assessing the presence of antigen-specific cytotoxic CD8+ T cells using MHC class-I (MHC-I) multimer technology. Previously we have designed conditional peptides for HLA-A*02:01, H-2K b and HLA-E that form stable peptide-MHC-I-complexes at low temperatures and dissociate when exposed to a defined elevated temperature. The resulting conditional MHC-I complex can easily and without additional handling be exchanged with a peptide of interest, allowing to exchange peptides in a ready-to-use multimer and a high-throughput manner. Here we present data that this peptide-exchange technology is a general applicable, ready-to-use and fast approach to load many different peptides in MHC-I multimers for alleles of the HLA-A, HLA-B and HLA-C loci. We describe the development of conditional peptides for HLA-A*03:01, HLA-A*11:01, HLA-B*07:02 and HLA-C*07:02 that only form stable peptide-MHC-I complexes at low temperatures, allowing peptide exchange at higher defined temperature. We document the ease and flexibility of this technology by monitoring CD8+ T cell responses to virus-specific peptide-MHC complexes in patients. Graphical abstract Highlights T cell immunity relies on antigen-specific CD8+ T cells recognizing peptide MHC-I complexes. Establishing temperature-based peptide exchange across multiple HLA alleles, resulting in a robust, easy, and fast system to generate peptide MHC-I complexes. Temperature-based MHC class-I multimer demonstrate applicability across major MHC-I gene families for monitoring CD8+ T cell responses. Easy high-throughput peptide exchange potential, enhancing clinical utility of MHC multimer technology.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Nature Communications on 3 January 2024 by Sankhala, R. S., Lal, K. G., et al.
The repeat emergence of SARS-CoV-2 variants of concern (VoC) with decreased susceptibility to vaccine-elicited antibodies highlights the need to develop next-generation vaccine candidates that confer broad protection. Here we describe the antibody response induced by the SARS-CoV-2 Spike Ferritin Nanoparticle (SpFN) vaccine candidate adjuvanted with the Army Liposomal Formulation including QS21 (ALFQ) in non-human primates. By isolating and characterizing several monoclonal antibodies directed against the Spike Receptor Binding Domain (RBD), N-Terminal Domain (NTD), or the S2 Domain, we define the molecular recognition of vaccine-elicited cross-reactive monoclonal antibodies (mAbs) elicited by SpFN. We identify six neutralizing antibodies with broad sarbecovirus cross-reactivity that recapitulate serum polyclonal antibody responses. In particular, RBD mAb WRAIR-5001 binds to the conserved cryptic region with high affinity to sarbecovirus clades 1 and 2, including Omicron variants, while mAb WRAIR-5021 offers complete protection from B.1.617.2 (Delta) in a murine challenge study. Our data further highlight the ability of SpFN vaccination to stimulate cross-reactive B cells targeting conserved regions of the Spike with activity against SARS CoV-1 and SARS-CoV-2 variants.
© 2024. The Author(s).
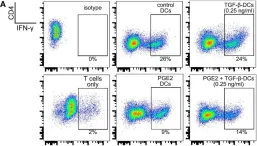
In Front Immunol on 11 July 2018 by Remes Lenicov, F., Paletta, A. L., et al.
Fig.6.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1