The rapid onset of innate immune defenses is critical for early control of viral replication in an infected host and yet it can also lead to irreversible tissue damage, especially in the respiratory tract. Sensitive regulators must exist that modulate inflammation, while controlling the infection. In the present study, we identified acetylcholine (ACh)-producing B cells as such early regulators. B cells are the most prevalent ACh-producing leukocyte population in the respiratory tract demonstrated with choline acetyltransferase (ChAT)-green fluorescent protein (GFP) reporter mice, both before and after infection with influenza A virus. Mice lacking ChAT in B cells, disabling their ability to generate ACh (ChatBKO), but not those lacking ChAT in T cells, significantly, selectively and directly suppressed α7-nicotinic-ACh receptor-expressing interstitial, but not alveolar, macrophage activation and their ability to secrete tumor necrosis factor (TNF), while better controlling virus replication at 1 d postinfection. Conversely, TNF blockade via monoclonal antibody treatment increased viral loads at that time. By day 10 of infection, ChatBKO mice showed increased local and systemic inflammation and reduced signs of lung epithelial repair despite similar viral loads and viral clearance. Thus, B cells are key participants of an immediate early regulatory cascade that controls lung tissue damage after viral infection, shifting the balance toward reduced inflammation at the cost of enhanced early viral replication.
© 2025. The Author(s).
Product Citations: 12
B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine.
In Nature Immunology on 1 May 2025 by Cembellin-Prieto, A., Luo, Z., et al.
-
Immunology and Microbiology
Altered X-chromosome inactivation predisposes to autoimmunity.
In Science Advances on 3 May 2024 by Huret, C., Ferrayé, L., et al.
In mammals, males and females show marked differences in immune responses. Males are globally more sensitive to infectious diseases, while females are more susceptible to systemic autoimmunity. X-chromosome inactivation (XCI), the epigenetic mechanism ensuring the silencing of one X in females, may participate in these sex biases. We perturbed the expression of the trigger of XCI, the noncoding RNA Xist, in female mice. This resulted in reactivation of genes on the inactive X, including members of the Toll-like receptor 7 (TLR7) signaling pathway, in monocyte/macrophages and dendritic and B cells. Consequently, female mice spontaneously developed inflammatory signs typical of lupus, including anti-nucleic acid autoantibodies, increased frequencies of age-associated and germinal center B cells, and expansion of monocyte/macrophages and dendritic cells. Mechanistically, TLR7 signaling is dysregulated in macrophages, leading to sustained expression of target genes upon stimulation. These findings provide a direct link between maintenance of XCI and female-biased autoimmune manifestations and highlight altered XCI as a cause of autoimmunity.
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
In Nature Communications on 5 July 2023 by Lam, J. H. & Baumgarth, N.
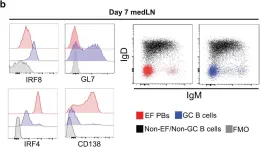
Extrafollicular plasmablast responses (EFRs) are considered to generate antibodies of low affinity that offer little protection from infections. Paradoxically, high avidity antigen-B cell receptor engagement is thought to be the main driver of B cell differentiation, whether in EFRs or slower-developing germinal centers (GCs). Here we show that influenza infection rapidly induces EFRs, generating protective antibodies via Toll-like receptor (TLR)-mediated mechanisms that are both B cell intrinsic and extrinsic. B cell-intrinsic TLR signals support antigen-stimulated B cell survival, clonal expansion, and the differentiation of B cells via induction of IRF4, the master regulator of B cell differentiation, through activation of NF-kB c-Rel. Provision of sustained TLR4 stimulation after immunization shifts the fate of virus-specific B cells towards EFRs instead of GCs, prompting rapid antibody production and improving their protective capacity over antigen/alum administration alone. Thus, inflammatory signals act as B cell fate-determinants for the rapid generation of protective antiviral extrafollicular responses.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
SREBP signaling is essential for effective B cell responses.
In Nature Immunology on 1 February 2023 by Luo, W., Adamska, J. Z., et al.
Our previous study using systems vaccinology identified an association between the sterol regulatory binding protein (SREBP) pathway and humoral immune response to vaccination in humans. To investigate the role of SREBP signaling in modulating immune responses, we generated mice with B cell- or CD11c+ antigen-presenting cell (APC)-specific deletion of SCAP, an essential regulator of SREBP signaling. Ablation of SCAP in CD11c+ APCs had no effect on immune responses. In contrast, SREBP signaling in B cells was critical for antibody responses, as well as the generation of germinal centers,memory B cells and bone marrow plasma cells. SREBP signaling was required for metabolic reprogramming in activated B cells. Upon mitogen stimulation, SCAP-deficient B cells could not proliferate and had decreased lipid rafts. Deletion of SCAP in germinal center B cells using AID-Cre decreased lipid raft content and cell cycle progression. These studies provide mechanistic insights coupling sterol metabolism with the quality and longevity of humoral immunity.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
-
Immunology and Microbiology
In The Journal of Immunology on 15 November 2022 by Matter, A. L., Liggitt, D., et al.
Multiple sclerosis (MS) is an inflammatory, demyelinating CNS disease believed to be mediated by CD4 T cells specific for CNS self-antigens. CD8 T cells are also implicated in MS but their function is not well understood. MS lesions are heterogeneous and may reflect variation in the contribution of different types of lymphocytes. Understanding how lymphocytes with different effector functions contribute to MS is essential to develop effective therapies. We investigated how T cells expressing an MHC class I-restricted transgenic TCR specific for myelin basic protein (MBP) contribute to CNS autoimmunity using the mouse model of MS, experimental autoimmune encephalomyelitis. Virus infection triggered cytotoxic TCR-transgenic CD8 T cells to initiate acute experimental autoimmune encephalomyelitis in an IFN-γ- and perforin-dependent manner. Unexpectedly, spontaneous CNS autoimmunity developed in the TCR-transgenic mice that was accelerated by IFN-γ-deficiency. Spontaneous disease was associated with CD4 T cells that develop via endogenous TCR rearrangements but retain specificity for the MHC class I-restricted MBP epitope. The CD4 T cells produced TNF-α without other inflammatory cytokines and caused lesions with striking similarity to active MS lesions. Surprisingly, B cells were the predominant cell type that cross-presented MBP, and their depletion halted disease progression. This work provides a new model of spontaneous CNS autoimmunity with unique similarities to MS that is mediated by T cells with a distinct effector phenotype.
Copyright © 2022 by The American Association of Immunologists, Inc.
-
Immunology and Microbiology
In Nat Commun on 5 July 2023 by Lam, J. H. & Baumgarth, N.
Fig.1.B

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1