Adenosine (Ado) mediates immune suppression in the tumor microenvironment and exhausted CD8+ CAR-T cells express CD39 and CD73, which mediate proximal steps in Ado generation. Here, we sought to enhance CAR-T cell potency by knocking out CD39, CD73, or adenosine receptor 2a (A2aR) but observed only modest effects. In contrast, overexpression of Ado deaminase (ADA-OE), which metabolizes Ado to inosine (INO), induced stemness and enhanced CAR-T functionality. Similarly, CAR-T cell exposure to INO augmented function and induced features of stemness. INO induced profound metabolic reprogramming, diminishing glycolysis, increasing mitochondrial and glycolytic capacity, glutaminolysis and polyamine synthesis, and reprogrammed the epigenome toward greater stemness. Clinical scale manufacturing using INO generated enhanced potency CAR-T cell products meeting criteria for clinical dosing. These results identify INO as a potent modulator of CAR-T cell metabolism and epigenetic stemness programming and deliver an enhanced potency platform for cell manufacturing.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 4
Inosine induces stemness features in CAR-T cells and enhances potency.
In Cancer Cell on 12 February 2024 by Klysz, D. D., Fowler, C., et al.
-
Cancer Research
-
Immunology and Microbiology
In International Journal of Molecular Sciences on 9 August 2023 by Rochín-Hernández, L. J., Jiménez-Acosta, M. A., et al.
Alzheimer's disease (AD), the most common neurodegenerative disease and the first cause of dementia worldwide, has no effective treatment, and its pathological mechanisms are not yet fully understood. We conducted this study to explore the proteomic differences associated with Familial Alzheimer's Disease (FAD) in olfactory ecto-mesenchymal stem cells (MSCs) derived from PSEN1 (A431E) mutation carriers compared with healthy donors paired by age and gender through two label-free liquid chromatography-mass spectrometry approaches. The first analysis compared carrier 1 (patient with symptoms, P1) and its control (healthy donor, C1), and the second compared carrier 2 (patient with pre-symptoms, P2) with its respective control cells (C2) to evaluate whether the protein alterations presented in the symptomatic carrier were also present in the pre-symptom stages. Finally, we analyzed the differentially expressed proteins (DEPs) for biological and functional enrichment. These proteins showed impaired expression in a stage-dependent manner and are involved in energy metabolism, vesicle transport, actin cytoskeleton, cell proliferation, and proteostasis pathways, in line with previous AD reports. Our study is the first to conduct a proteomic analysis of MSCs from the Jalisco FAD patients in two stages of the disease (symptomatic and presymptomatic), showing these cells as a new and excellent in vitro model for future AD studies.
-
FC/FACS
-
Homo sapiens (Human)
-
Neuroscience
-
Stem Cells and Developmental Biology
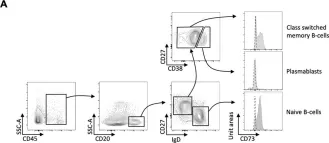
Tumor Infiltration with CD20+CD73+ B Cells Correlates with Better Outcome in Colorectal Cancer.
In International Journal of Molecular Sciences on 5 May 2022 by Hansen, F. J., Wu, Z., et al.
Immunotherapy has become increasingly important in the treatment of colorectal cancer (CRC). Currently, CD73, also known as ecto-5'-nucleotidase (NT5E), has gained considerable interest as a potential therapeutic target. CD73 is one of the key enzymes catalyzing the conversion of extracellular ATP into adenosine, which in turn exerts potent immune suppressive effects. However, the role of CD73 expression on various cell types within the CRC tumor microenvironment remains unresolved. The expression of CD73 on various cell types has been described recently, but the role of CD73 on B-cells in CRC remains unclear. Therefore, we analyzed CD73 on B-cells, especially on tumor-infiltrating B-cells, in paired tumor and adjacent normal tissue samples from 62 eligible CRC patients. The highest expression of CD73 on tumor-infiltrating B-cells was identified on class-switched memory B-cells, followed by naive B-cells, whereas no CD73 expression was observed on plasmablasts. Clinicopathological correlation analysis revealed that higher CD73+ B-cells infiltration in the CRC tumors was associated with better overall survival. Moreover, metastasized patients showed a significantly decreased number of tumor-infiltrating CD73+ B-cells. Finally, neoadjuvant therapy correlated with reduced CD73+ B-cell numbers and CD73 expression on B-cells in the CRC tumors. As promising new immune therapies are being developed, the role of CD73+ B-cells and their subsets in the development of colorectal cancer should be further explored to find new therapeutic options.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1.
In Investigative Ophthalmology & Visual Science on 1 October 2017 by Eslani, M., Putra, I., et al.
To evaluate the angiogenic properties of corneal derived mesenchymal stromal cells (Co-MSC).
Co-MSCs were extracted from human cadaver, and wild-type (C57BL/6J) and SERPINF1-/- mice corneas. The MSC secretome was collected in a serum-free medium. Human umbilical vein endothelial cell (HUVEC) tube formation and fibrin gel bead assay (FIBA) sprout formation were used to assess the angiogenic properties of Co-MSC secretome. Complete corneal epithelial debridement was used to induce corneal neovascularization in wild-type mice. Co-MSCs embedded in fibrin gel was applied over the debrided cornea to evaluate the angiogenic effects of Co-MSCs in vivo. Immunoprecipitation was used to remove soluble fms-like tyrosine kinase-1 (sFLT-1) and pigment epithelium-derived factor (PEDF, SERPINF1 gene) from the Co-MSC secretome.
Co-MSC secretome significantly inhibited HUVECs tube and sprout formation. Co-MSCs from different donors consistently contained high levels of antiangiogenic factors including sFLT-1 and PEDF; and low levels of the angiogenic factor VEGF-A. In vivo, application of Co-MSCs to mouse corneas after injury prevented the development of corneal neovascularization. Removing PEDF or sFLT-1 from the secretome significantly diminished the antiangiogenic effects of Co-MSCs. Co-MSCs isolated from SERPINF1-/- mice had significantly reduced antiangiogenic effects compared to SERPINF1+/+ (wild-type) Co-MSCs.
These results illustrate the direct antiangiogenic properties of Co-MSCs, the importance of sFLT-1 and PEDF, and their potential clinical application for preventing pathologic corneal neovascularization.
-
Neuroscience
In Int J Mol Sci on 5 May 2022 by Hansen, F. J., Wu, Z., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 1