Immunotherapy has transformed cancer treatment but benefits only some patients, and predictive biomarkers are lacking. One correlate of response is the reinvigoration of a subset of CD8 T cells that have an exhausted phenotype and impaired functionality. To develop effective therapies, reproducible models are required to identify candidate target genes that enable reversal of T cell exhaustion. Here, we describe an in vitro model by chronically stimulating T cells with their cognate antigen, followed by temporal phenotypic characterization. This model recapitulates many critical hallmarks of exhaustion, including expression of canonical surface markers, impaired proliferation, reduced cytokine production, decreased cytotoxic granule release, and metabolic alterations. Two in vivo models validate these results and establish a gene signature shared by in vitro and in vivo exhausted states. Critically, this signature is observed in tumor infiltrating T cells from multiple human tumor types, validating the translational potential of this model for discovering therapies.
© 2025 The Authors.
Product Citations: 53
Simulating CD8 T cell exhaustion: A comprehensive approach.
In IScience on 18 July 2025 by Manrique-Rincón, A. J., Foster, B., et al.
-
Immunology and Microbiology
Inhibition of ENT1 relieves intracellular adenosine-mediated T cell suppression in cancer.
In Nature Immunology on 1 June 2025 by Sanders, T., Nabel, C. S., et al.
The benefit of immune checkpoint blockade for cancer therapy is limited to subsets of patients because of factors including the accumulation of immunosuppressive metabolites, such as adenosine, within tumors. Pharmacological inhibition of adenosine generation and signaling is an active area of clinical investigation, but only limited clinical benefit has been reported. Here, we show that adenosine suppresses anti-cancer T cell responses following uptake into activated T cells by equilibrative nucleoside transporter 1 (ENT1) and inhibition of de novo pyrimidine nucleotide synthesis. We identify EOS301984 as a potent ENT1 antagonist that restores pyrimidine levels in activated T cells in adenosine-rich environments, resulting in enhanced tumor cell killing by memory T cells and increased ex vivo expansion of functional human tumor-infiltrating lymphocytes. A combination of EOS301984 with anti-PD-1 led to synergistic control of tumor growth in a humanized mouse model of triple-negative breast cancer. ENT1 inhibition, therefore, augments anti-cancer immune responses through the restoration of pyrimidine nucleotide synthesis in T cells suppressed by adenosine.
© 2025. The Author(s).
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
The MYC/TXNIP axis mediates NCL-Suppressed CD8+T cell immune response in lung adenocarcinoma.
In Molecular Medicine on 9 May 2025 by Xiao, D., Chen, T., et al.
Lung adenocarcinoma is a deadly malignancy with immune evasion playing a key role in tumor progression. Glucose metabolism is crucial for T cell function, and the nucleolar protein NCL may influence T cell glucose metabolism. This study aims to investigate NCL's role in T cell glucose metabolism and immune evasion by lung adenocarcinoma cells.
Utilizing single-cell RNA sequencing (scRNA-seq) data from the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA), we analyzed cell clustering, annotation, and prognosis. In vitro experiments involved manipulating NCL expression in CD8+ T cells to study immune function and glucose metabolism. In vivo studies using an orthotopic transplant mouse model monitored NCL's impact on CD8+ T cell glucose metabolism and anti-tumor immune function.
NCL was associated with T cell dysfunction and glucose metabolism. NCL silencing enhanced CD8+ T cell glucose metabolism, cytotoxicity, and infiltration, while NCL overexpression had the opposite effect. NCL overexpression relieved MYC-mediated transcriptional repression of TXNIP, reducing CD8+ T cell glucose metabolism. In vivo, NCL inhibited CD8+ T cell glucose metabolism through the MYC/TXNIP axis, hindering anti-tumor immune function.
NCL overexpression suppresses CD8+ T cell glucose metabolism and anti-tumor immune function, promoting lung adenocarcinoma progression via the MYC/TXNIP axis.
© 2025. The Author(s).
-
FC/FACS
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
Targeted editing of CCL5 with CRISPR-Cas9 nanoparticles enhances breast cancer immunotherapy.
In Apoptosis : An International Journal On Programmed Cell Death on 1 April 2025 by Yan, W., Wang, S., et al.
Breast cancer remains one of the leading causes of cancer-related mortality among women worldwide. Immunotherapy, a promising therapeutic approach, often faces challenges due to the immunosuppressive tumor microenvironment. This study explores the innovative use of CRISPR-Cas9 technology in conjunction with FCPCV nanoparticles to target and edit the C-C Motif Chemokine Ligand 5 (CCL5) gene, aiming to improve the efficacy of breast cancer immunotherapy. Single-cell RNA sequencing (scRNA-seq) and TCGA-BRCA data identified CCL5 as a key immune-related gene in breast cancer. Using CRISPR-Cas9, sgRNA targeting CCL5 was designed and delivered to breast cancer cells and humanized mouse models via FCPCV nanoparticles. In vitro experiments demonstrated that FCPCV nanoparticles effectively silenced CCL5, enhanced CD8+ T cell activity, and increased the production of cytokines such as IFN-γ, TNF-α, and GZMB. In vivo studies revealed significant tumor suppression, improved immune microenvironment, and increased CD8+/CD4+ ratios in treated mice, without notable toxic side effects. These findings highlight the potential of CRISPR-Cas9 nanoparticle-mediated gene editing as a novel strategy for enhancing breast cancer immunotherapy, providing a new direction for personalized and effective cancer treatment.
© 2024. The Author(s).
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Transferrin Disassociates TCR from CD3 Signaling Apparatus to Promote Metastasis.
In Research (Washington, D.C.) on 15 January 2025 by Cheng, R., Tang, X., et al.
Immune recognition and activation by the peptide-laden major histocompatibility complex-T cell receptor (TCR)-CD3 complex is essential for anti-tumor immunity. Tumors may escape immune surveillance by dissembling the complex. Here, we report that transferrin, which is overexpressed in patients with liver metastasis, disassociates TCR from the CD3 signaling apparatus by targeting the constant domain (CD) of T cell receptor α (TCRα), consequently suppresses T cell activation, and inhibits anti-metastatic and anti-tumor immunity. In mouse models of melanoma and lymphoma, transferrin overexpression exacerbates liver metastasis, while its knockdown, antibody, designed peptides, and CD mutation interfering with transferrin-TCRα interaction inhibit metastasis. This work reveals a novel strategy of tumor evasion of immune surveillance by blocking the coupling between TCRs and the CD3 signaling apparatus to suppress TCR activation. Given the conservation of CD and transferrin up-regulation in metastatic tumors, the strategy might be a common metastatic mechanism. Targeting transferrin-TCRα holds promise for anti-metastatic treatment.
Copyright © 2025 Ruomei Cheng et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
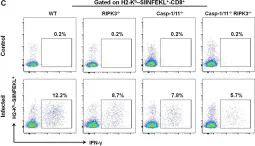
In Front Immunol on 25 April 2020 by Rana, A., de Almeida, F. C., et al.
Fig.5.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1