This article discusses methods to assess invariant natural killer T (iNKT) cell subsets isolated from the thymus, as well as the spleen, the liver, and the lung. iNKT cells can be subdivided in distinct, functional subsets based on the transcription factors they express and the cytokines they produce to regulate the immune response. Basic Protocol 1 focuses on characterizing murine iNKT subsets ex vivo by flow cytometry by evaluating the expression of lineage-specifying transcription factors such as PLZF and RORγt. The Alternate Protocol describes a detailed approach to define subsets based on expression of surface markers. This approach can be very useful for maintaining the subsets alive, without fixing them, in order to isolate them for downstream molecular assays such as DNA/RNA isolation, genome-wide analysis to assess gene expression (such as RNA-seq), assessment of chromatin accessibility (for instance, by ATAC-seq), and assessment of DNA methylation by whole-genome bisulfite sequencing. Basic Protocol 2 describes the functional characterization of iNKT cells, which are activated in vitro with PMA and ionomycin for a short period of time and subsequently stained and characterized for production of cytokines, such as IFNγ and IL-4, by flow cytometry. Basic Protocol 3 describes the process of activating iNKT cells in vivo using α-galactosyl-ceramide, a lipid that can be recognized specifically by iNKT cells, allowing assessment of their functionality in vivo. Cells are then isolated and directly stained for cytokine secretion. © 2023 Wiley Periodicals LLC. Basic Protocol 1: Identifying iNKT cell subsets based on transcription factor expression by flow cytometry Alternate Protocol: Identifying iNKT cell subsets based on surface marker expression by flow cytometry Basic Protocol 2: iNKT cell functional characterization based on in vitro activation and assessment of cytokine secretion Basic Protocol 3: iNKT cell in vivo activation and assessment of cytokine secretion by flow cytometry.
© 2023 Wiley Periodicals LLC.
Product Citations: 14
Defining iNKT Cell Subsets and Their Function by Flow Cytometry.
In Current Protocols on 1 July 2023 by Gioulbasani, M. & Tsagaratou, A.
-
Mus musculus (House mouse)
In Nature Communications on 3 December 2022 by Hackstein, C. P., Costigan, D., et al.
Interactions with commensal microbes shape host immunity on multiple levels and play a pivotal role in human health and disease. Tissue-dwelling, antigen-specific T cells are poised to respond to local insults, making their phenotype important in the relationship between host and microbes. Here we show that MHC-II restricted, commensal-reactive T cells in the colon of both humans and mice acquire transcriptional and functional characteristics associated with innate-like T cells. This cell population is abundant and conserved in the human and murine colon and endowed with polyfunctional effector properties spanning classic Th1- and Th17-cytokines, cytotoxic molecules, and regulators of epithelial homeostasis. T cells with this phenotype are increased in ulcerative colitis patients, and their presence aggravates pathology in dextran sodium sulphate-treated mice, pointing towards a pathogenic role in colitis. Our findings add to the expanding spectrum of innate-like immune cells positioned at the frontline of intestinal immune surveillance, capable of acting as sentinels of microbes and the local cytokine milieu.
© 2022. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Cell Reports on 29 November 2022 by Watanabe, M., Celli, S., et al.
Invariant natural killer T (iNKT) cell development in the thymus depends on T cell receptor recognition of CD1d ligand on CD4/CD8 double-positive thymocytes. We previously reported that B7-CD28 co-stimulation is required for thymic iNKT cell development, but the cellular and molecular mechanisms underlying this co-stimulatory requirement are not understood. Here we report that CD28 expression on CD1d-expressing antigen-presenting T cells is required for thymic iNKT cell development. Mechanistically, antigen-presenting T cells provide co-stimulation through an unconventional mechanism, acquiring B7 molecules via CD28-dependent trogocytosis from B7-expressing thymic epithelial cells, dendritic cells, and B cells and providing critical B7 co-stimulation to developing iNKT cells. Thus, the present study demonstrates a mechanism of B7 co-stimulation in thymic T cell development by antigen-presenting T cells.
Published by Elsevier Inc.
-
Immunology and Microbiology
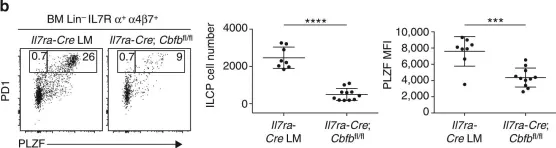
In Nature Immunology on 1 October 2021 by Stehle, C., Rückert, T., et al.
The generation of lymphoid tissues during embryogenesis relies on group 3 innate lymphoid cells (ILC3) displaying lymphoid tissue inducer (LTi) activity and expressing the master transcription factor RORγt. Accordingly, RORγt-deficient mice lack ILC3 and lymphoid structures, including lymph nodes (LN). Whereas T-bet affects differentiation and functions of ILC3 postnatally, the role of T-bet in regulating fetal ILC3 and LN formation remains completely unknown. Using multiple mouse models and single-cell analyses of fetal ILCs and ILC progenitors (ILCP), here we identify a key role for T-bet during embryogenesis and show that its deficiency rescues LN formation in RORγt-deficient mice. Mechanistically, T-bet deletion skews the differentiation fate of fetal ILCs and promotes the accumulation of PLZFhi ILCP expressing central LTi molecules in a RORα-dependent fashion. Our data unveil an unexpected role for T-bet and RORα during embryonic ILC function and highlight that RORγt is crucial in counteracting the suppressive effects of T-bet.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Nature Communications on 4 March 2021 by Murray, M. P., Engel, I., et al.
Invariant natural killer T cells (iNKT cells) differentiate into thymic and peripheral NKT1, NKT2 and NKT17 subsets. Here we use RNA-seq and ATAC-seq analyses and show iNKT subsets are similar, regardless of tissue location. Lung iNKT cell subsets possess the most distinct location-specific features, shared with other innate lymphocytes in the lung, possibly consistent with increased activation. Following antigenic stimulation, iNKT cells undergo chromatin and transcriptional changes delineating two populations: one similar to follicular helper T cells and the other NK or effector like. Phenotypic analysis indicates these changes are observed long-term, suggesting that iNKT cells gene programs are not fixed, but they are capable of chromatin remodeling after antigen to give rise to additional subsets.
-
Immunology and Microbiology
In Nat Commun on 16 October 2017 by Mao, A. P., Ishizuka, I. E., et al.
Fig.8.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2
In Nat Commun on 16 October 2017 by Mao, A. P., Ishizuka, I. E., et al.
Fig.8.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2