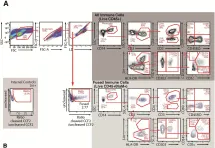
Reverse genetics approaches in mice are widely used to understand gene functions and their aberrations in diseases. However, limitations exist in translating findings from animal models to human physiology. Humanized mice provide a powerful bridge to understanding human physiology and mechanisms of disease pathogenesis while maintaining the feasibility of working with small animals. Methods for generating humanized mouse models that allow scientists to probe contributions of particular genes have been rudimentary. Here, we established an efficient method for generating genetically modified human cord blood-derived CD34+ cells for transplantation, resulting in humanized mice with near-complete loss of specific gene expression by the human immune system. Mice transplanted with Cas9-edited human CD34+ cells recapitulate functional consequences of specific gene losses in the human immune system. Our approach enables targeted gene knockouts in humanized mice, offering a valuable tool for human gene function studies in vivo.
Product Citations: 62
Establishment of a reverse genetics system for studying human immune functions in mice.
In Science Advances on 11 July 2025 by Pal, P., Gao, S., et al.
-
Genetics
-
Immunology and Microbiology
In Nature Communications on 8 April 2025 by Lim, J. J., Jones, C. M., et al.
αβ T cell receptors (TCR) co-recognise peptide (p) antigens that are presented by major histocompatibility complex (MHC) molecules. While marked variations in TCR-p-MHC docking topologies have been observed from structural studies, the co-recognition paradigm has held fast. Using HLA-DQ2.5-peptide tetramers, here we identify a TRAV12-1+-TRBV5-1+ G9 TCR from human peripheral blood that binds HLA-DQ2.5 in a peptide-agnostic manner. The crystal structures of TCR-HLA-DQ2.5-peptide complexes show that the G9 TCR binds HLA-DQ2.5 in a reversed docking topology without contacting the peptide, with the TCR contacting the β1 region of HLA-DQ2.5 and distal from the peptide antigen binding cleft. High-throughput screening of HLA class I and II molecules finds the G9 TCR to be pan-HLA-DQ2 reactive, with leucine-55 of HLA-DQ2.5 being a key determinant underpinning G9 TCR specificity excluding other HLA-II allomorphs. Consistent with the functional assays, the interactions of the G9 TCR and HLA-DQ2.5 precludes CD4 binding, thereby impeding T cell activation. Collectively, we describe a naturally selected αβTCR from human peripheral blood that deviates from the TCR-p-MHC co-recognition paradigm.
© 2025. The Author(s).
-
Immunology and Microbiology
In Scientific Reports on 17 February 2025 by Sytsma, B. J., Allain, V., et al.
Adoptive chimeric antigen receptor T-cell (CAR-T) therapy is transformative and approved for hematologic malignancies. It is also being developed for the treatment of solid tumors, autoimmune disorders, heart disease, and aging. Despite unprecedented clinical outcomes, CAR-T and other engineered cell therapies face a variety of manufacturing and safety challenges. Traditional methods, such as lentivirus transduction and electroporation, result in random integration or cause significant cellular damage, which can limit the safety and efficacy of engineered cell therapies. We present hydroporation as a gentle and effective alternative for intracellular delivery. Hydroporation resulted in 1.7- to 2-fold higher CAR-T yields compared to electroporation with superior cell viability and recovery. Hydroporated cells exhibited rapid proliferation, robust target cell lysis, and increased pro-inflammatory and regulatory cytokine secretion in addition to improved CAR-T yield by day 5 post-transfection. We demonstrate that scaled-up hydroporation can process 5 × 108 cells in less than 10 s, showcasing the platform as a viable solution for high-yield CAR-T manufacturing with the potential for improved therapeutic outcomes.
© 2025. The Author(s).
-
FC/FACS
-
Immunology and Microbiology
In Clinical and Experimental Immunology on 21 January 2025 by Radziszewska, A., Peckham, H., et al.
Juvenile systemic lupus erythematosus (JSLE) is an autoimmune condition which causes significant morbidity in children and young adults and is more severe in its presentation than adult-onset SLE. While many aspects of immune dysfunction have been studied extensively in adult-onset SLE, there is limited and contradictory evidence of how cytotoxic CD8+ T cells contribute to disease pathogenesis and studies exploring cytotoxicity in JSLE are virtually non-existent. Here, we report that CD8+ T cell cytotoxic capacity is reduced in JSLE versus healthy controls, irrespective of treatment or disease activity. Transcriptomic and serum metabolomic analysis identified that this reduction in cytotoxic CD8+ T cells in JSLE was associated with upregulated type I interferon (IFN) signalling, mitochondrial dysfunction, and metabolic disturbances when compared to controls. Greater interrogation of the influence of these pathways on altered cytotoxic CD8+ T cell function demonstrated that JSLE CD8+ T cells had enlarged mitochondria and enhanced sensitivity to IFN-α leading to selective apoptosis of effector memory (EM) CD8+ T cells, which are enriched for cytotoxic mediator-expressing cells. This process ultimately contributes to the observed reduction in CD8+ T cell cytotoxicity in JSLE, reinforcing the growing evidence that mitochondrial dysfunction is a key pathogenic factor affecting multiple immune cell populations in type I IFN-driven rheumatic diseases.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Immunology.
-
Homo sapiens (Human)
-
Cell Biology
-
Immunology and Microbiology
Mechanotransduction governs CD40 function and underlies X-linked hyper-IgM syndrome.
In Science Advances on 15 November 2024 by Choi, H. K., Travaglino, S., et al.
B cell maturation depends on cognate interactions between the T and B cells. Upon interaction with CD40 ligand (CD40L) on T cells, CD40 delivers costimulatory signals alongside B cell antigen receptor (BCR) signaling to regulate affinity maturation and antibody class switch. Mutations affecting CD40-CD40L interactions cause abnormal antibody responses in immunodeficiencies known as X-linked hyper-IgM syndrome (X-HIgM). Here, we study the CD40-mediated mechanotransduction in B cells, which likely occurs during their physical contacts with T cells. We found that CD40 forms catch bond with CD40L that lasts longer at larger forces, both B and T cells exert tension on CD40-CD40L bonds, and force enhances CD40 signaling and antibody class switch. X-HIgM CD40L mutations impair catch bond formation, suppress endogenous tension, and reduce force-enhanced CD40 signaling, leading to deficiencies in antibody class switch. Our findings highlight the role of mechanotransduction in CD40 function and provide insights into the mechanisms underlying X-HIgM syndrome.
In PLoS One on 23 August 2019 by Cavrois, M., Hilton, J. F., et al.
Fig.3.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS One on 23 August 2019 by Cavrois, M., Hilton, J. F., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2