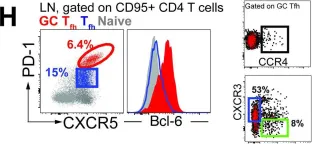
CD4 T follicular helper cells (Tfh) are essential for establishing serological memory and have distinct helper attributes that impact both the quantity and quality of the antibody response. Insights into Tfh subsets that promote antibody persistence and functional capacity can critically inform vaccine design. Based on the Tfh profiles evoked by the live attenuated measles virus vaccine, renowned for its ability to establish durable humoral immunity, we investigated the potential of a Tfh1/17 recall response during the boost phase to enhance persistence of HIV-1 Envelope (Env) antibodies in rhesus macaques. Using a DNA-prime encoding gp160 antigen and Tfh polarizing cytokines (interferon protein-10 (IP-10) and interleukin-6 (IL-6)), followed by a gp140 protein boost formulated in a cationic liposome-based adjuvant (CAF01), we successfully generated germinal center (GC) Tfh1/17 cells. In contrast, a similar DNA-prime (including IP-10) followed by gp140 formulated with monophosphoryl lipid A (MPLA) +QS-21 adjuvant predominantly induced GC Tfh1 cells. While the generation of GC Tfh1/17 cells with CAF01 and GC Tfh1 cells with MPLA +QS-21 induced comparable peak Env antibodies, the latter group demonstrated significantly greater antibody concentrations at week 8 after final immunization which persisted up to 30 weeks (gp140 IgG ng/ml- MPLA; 5500; CAF01, 2155; p<0.05). Notably, interferon γ+Env-specific Tfh responses were consistently higher with gp140 in MPLA +QS-21 and positively correlated with Env antibody persistence. These findings suggest that vaccine platforms maximizing GC Tfh1 induction promote persistent Env antibodies, important for protective immunity against HIV.
© 2023, Verma, Hawes et al.
Product Citations: 5
Tailoring Tfh profiles enhances antibody persistence to a clade C HIV-1 vaccine in rhesus macaques.
In eLife on 22 February 2024 by Verma, A., Hawes, C. E., et al.
-
FC/FACS
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 1 July 2020 by Wang, Z., Zhao, M., et al.
T follicular helper (Tfh) cells are indispensable for the formation of germinal center (GC) reactions, whereas T follicular regulatory (Tfr) cells inhibit Tfh-mediated GC responses. Aberrant activation of Tfh cells contributes substantially to the pathogenesis of autoimmune diseases, such as systemic lupus erythematosus (SLE). Nonetheless, the molecular mechanisms mitigating excessive Tfh cell differentiation are not fully understood. Herein we demonstrate that the adenovirus E4 promoter-binding protein (E4BP4) mediates a feedback loop and acts as a transcriptional brake to inhibit Tfh cell differentiation. Furthermore, we show that such an immunological mechanism is compromised in patients with SLE. Establishing mice with either conditional knockout (cKO) or knockin (cKI) of the E4bp4 gene in T cells reveals that E4BP4 strongly inhibits Tfh cell differentiation. Mechanistically, E4BP4 regulates Bcl6 transcription by recruiting the repressive epigenetic modifiers HDAC1 and EZH2. E4BP4 phosphorylation site mutants have limited capability with regard to inhibiting Tfh cell differentiation. In SLE, we detected impaired phosphorylation of E4BP4, finding that this compromised transcription factor is positively correlated with disease activity. These findings unveiled molecular mechanisms by which E4BP4 restrains Tfh cell differentiation, whose compromised function is associated with uncontrolled autoimmune reactions in SLE.
-
Immunology and Microbiology
In JCI Insight on 14 November 2019 by Jegaskanda, S., Andrews, S. F., et al.
Broadly neutralizing Abs targeting the HA stem can provide broad protection against different influenza subtypes, raising the question of how best to elicit such Abs. We have previously demonstrated that vaccination with pandemic live-attenuated influenza vaccine (pLAIV) establishes immune memory for HA head-specific Abs. Here, we determine the extent to which matched versus mismatched LAIV-inactivated subunit vaccine (IIV) prime-boost vaccination elicits stem-specific memory B cells and Abs. We vaccinated African green monkeys with H5N1 pLAIV-pIIV or H5N1 pLAIV followed by seasonal IIV (sIIV) or with H5N1 pLAIV alone and measured Abs and HA-specific B cell responses. While we observed an increase in stem-specific memory B cells, head-specific memory B cell responses were substantially higher than stem-specific responses and were dominant even following boost with mismatched IIV. Neutralizing Abs against heterologous influenza viruses were undetectable. Head-specific B cells from draining lymph nodes exhibited germinal center markers, while stem-specific B cells found in the spleen and peripheral blood did not. Thus, although mismatched prime-boost generated a pool of stem-specific memory B cells, head-specific B cells and serum Abs substantially dominated the immune response. These findings have implications for including full-length native HA in prime-boost strategies intended to induce stem-specific Abs for universal influenza vaccination.
-
Immunology and Microbiology
In Journal of Virology on 15 February 2019 by Helmold Hait, S., Vargas-Inchaustegui, D. A., et al.
T follicular helper (TFH) cells are fundamental in germinal center (GC) maturation and selection of antigen-specific B cells within secondary lymphoid organs. GC-resident TFH cells have been fully characterized in human immunodeficiency virus (HIV) infection. However, the role of GC TFH cells in GC B cell responses following various simian immunodeficiency virus (SIV) vaccine regimens in rhesus macaques (RMs) has not been fully investigated. We characterized GC TFH cells of RMs over the course of a mucosal/systemic vaccination regimen to elucidate GC formation and SIV humoral response generation. Animals were mucosally primed twice with replicating adenovirus type 5 host range mutant (Ad5hr)-SIV recombinants and systemically boosted with ALVAC-SIVM766Gag/Pro/gp120-TM and SIVM766&CG7V gD-gp120 proteins formulated in alum hydroxide (ALVAC/Env) or DNA encoding SIVenv/SIVGag/rhesus interleukin 12 (IL-12) plus SIVM766&CG7V gD-gp120 proteins formulated in alum phosphate (DNA&Env). Lymph nodes were biopsied in macaque subgroups prevaccination and at day 3, 7, or 14 after the 2nd Ad5hr-SIV prime and the 2nd vector/Env boost. Evaluations of GC TFH and GC B cell dynamics including correlation analyses supported a significant role for early GC TFH cells in providing B cell help during initial phases of GC formation. GC TFH responses at day 3 post-mucosal priming were consistent with generation of Env-specific memory B cells in GCs and elicitation of prolonged Env-specific humoral immunity in the rectal mucosa. GC Env-specific memory B cell responses elicited early post-systemic boosting correlated significantly with decreased viremia postinfection. Our results highlight the importance of early GC TFH cell responses for robust GC maturation and generation of long-lasting SIV-specific humoral responses at mucosal and systemic sites. Further investigation of GC TFH cell dynamics should facilitate development of an efficacious HIV vaccine.IMPORTANCE The modest HIV protection observed in the human RV144 vaccine trial associated antibody responses with vaccine efficacy. T follicular helper (TFH) cells are CD4+ T cells that select antibody secreting cells with high antigenic affinity in germinal centers (GCs) within secondary lymphoid organs. To evaluate the role of TFH cells in eliciting prolonged virus-specific humoral responses, we vaccinated rhesus macaques with a combined mucosal prime/systemic boost regimen followed by repeated low-dose intrarectal challenges with SIV, mimicking human exposure to HIV-1. Although the vaccine regimen did not prevent SIV infection, decreased viremia was observed in the immunized macaques. Importantly, vaccine-induced TFH responses elicited at day 3 postimmunization and robust GC maturation were strongly associated. Further, early TFH-dependent SIV-specific B cell responses were also correlated with decreased viremia. Our findings highlight the contribution of early vaccine-induced GC TFH responses to elicitation of SIV-specific humoral immunity and implicate their participation in SIV control.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
-
Immunology and Microbiology
In Journal of Virology on 1 May 2018 by Jegaskanda, S., Mason, R. D., et al.
Pandemic live attenuated influenza vaccines (pLAIV) prime subjects for a robust neutralizing antibody response upon subsequent administration of a pandemic inactivated subunit vaccine (pISV). However, a difference was not detected in H5-specific memory B cells in the peripheral blood between pLAIV-primed and unprimed subjects prior to pISV boost. To investigate the mechanism underlying pLAIV priming, we vaccinated groups of 12 African green monkeys (AGMs) with H5N1 pISV or pLAIV alone or H5N1 pLAIV followed by pISV and examined immunity systemically and in local draining lymph nodes (LN). The AGM model recapitulated the serologic observations from clinical studies. Interestingly, H5N1 pLAIV induced robust germinal center B cell responses in the mediastinal LN (MLN). Subsequent boosting with H5N1 pISV drove increases in H5-specific B cells in the axillary LN, spleen, and circulation in H5N1 pLAIV-primed animals. Thus, H5N1 pLAIV primes localized B cell responses in the MLN that are recalled systemically following pISV boost. These data provide mechanistic insights for the generation of robust humoral responses via prime-boost vaccination.IMPORTANCE We have previously shown that pandemic live attenuated influenza vaccines (pLAIV) prime for a rapid and robust antibody response on subsequent administration of inactivated subunit vaccine (pISV). This is observed even in individuals who had undetectable antibody (Ab) responses following the initial vaccination. To define the mechanistic basis of pLAIV priming, we turned to a nonhuman primate model and performed a detailed analysis of B cell responses in systemic and local lymphoid tissues following prime-boost vaccination with pLAIV and pISV. We show that the nonhuman primate model recapitulates the serologic observations from clinical studies. Further, we found that pLAIVs induced robust germinal center B cell responses in the mediastinal lymph node. Subsequent boosting with pISV in pLAIV-primed animals resulted in detection of B cells in the axillary lymph nodes, spleen, and peripheral blood. We demonstrate that intranasally administered pLAIV elicits a highly localized germinal center B cell response in the mediastinal lymph node that is rapidly recalled following pISV boost into germinal center reactions at numerous distant immune sites.
Copyright © 2018 American Society for Microbiology.
-
FC/FACS
-
Chlorocebus aethiops (Grivet monkey)
-
Immunology and Microbiology
In Elife on 22 February 2024 by Verma, A., Hawes, C. E., et al.
Fig.1.H

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 1