Programmed death-ligand 1 (PD-L1) is a key immune checkpoint molecule involved in tumor immune evasion. Its expression is highly heterogeneous across cancer types and subtypes, influencing therapeutic response. Understanding how different immunomodulatory agents influence PD-L1 expression in breast cancer cells could inform novel therapeutic strategies. This study aimed to investigate the temporal and dose-dependent effects of Bacillus Calmette-Guérin (BCG) and Toll-like receptor (TLR) agonists on PD-L1 expression in two breast cancer cell lines: MCF7 (luminal) and MDA-MB-231 (triple-negative).
MTT (thiazolyl blue tetrazolium bromide) assays were conducted to determine non-cytotoxic concentrations of the immunomodulatory agents: 25 µM IMQ (imiquimod), 10 µg PPG (peptidoglycan), 1 mg LPS (lipopolysaccharide), and two BCG doses (200 µg/mL and 800 µg/mL). Flow cytometry assessed anti-PD-L1 (CD274) antibody expression at 24- and 48 hours post-treatment.
In MCF7 cells, BCG induced a dose-dependent upregulation of PD-L1 at 24 hours, which was not sustained at 48 hours, while TLR agonists had minimal or slightly suppressive effects. In contrast, MDA-MB-231 cells exhibited a time-dependent modulation of PD-L1, with an increase at 24 hours followed by a reduction at 48 hours in response to BCG, while TLR agonists consistently decreased PD-L1 levels compared to controls.
These findings suggest distinct immunomodulatory responses between cancer subtypes, emphasizing the need for tailored approaches targeting the PD-1/PD-L1 axis. Further studies should explore the molecular mechanisms underlying these differential effects and assess the potential for combinatorial immunotherapeutic strategies in cancer.
© 2025 Barbosa et al.
Product Citations: 25
In International Journal of General Medicine on 1 July 2025 by Barbosa, G., de Godoy, M. C. X., et al.
-
FC/FACS
-
Cancer Research
Miltefosine reinvigorates exhausted T cells by targeting their bioenergetic state.
In Cell Reports Medicine on 17 December 2024 by Zhang, X., Zhang, C., et al.
T cell exhaustion presents a major challenge for the efficacy of both immune checkpoint inhibitors (ICBs) and chimeric antigen receptor T (CAR-T) cell immunotherapies. To address this issue, we generate hypofunctional CAR-T cells that imitate the exhaustion state. By screening a Food and Drug Administration (FDA)-approved small molecule library using this model, we identify miltefosine as a potent molecule that restores the impaired function of CAR-T cells in a PD-1/PD-L1-independent manner. Impressively, in the terminally exhausted state where PD-1 antibody treatment is ineffective, miltefosine still enhances CAR-T cell activity. Single-cell sequencing analysis reveals that miltefosine treatment significantly increases the population of effector cells. Mechanistically, miltefosine improves impaired glycolysis and oxidative phosphorylation in hypofunctional CAR-T cells. In both allogeneic and syngeneic tumor models, miltefosine effectively enhances the solid tumor clearance ability of CAR-T cells and T cells, demonstrating its potential as an effective immunotherapeutic drug.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Tuft cells act as regenerative stem cells in the human intestine.
In Nature on 1 October 2024 by Huang, L., Bernink, J. H., et al.
In mice, intestinal tuft cells have been described as a long-lived, postmitotic cell type. Two distinct subsets have been identified: tuft-1 and tuft-2 (ref. 1). By combining analysis of primary human intestinal resection material and intestinal organoids, we identify four distinct human tuft cell states, two of which overlap with their murine counterparts. We show that tuft cell development depends on the presence of Wnt ligands, and that tuft cell numbers rapidly increase on interleukin-4 (IL-4) and IL-13 exposure, as reported previously in mice2-4. This occurs through proliferation of pre-existing tuft cells, rather than through increased de novo generation from stem cells. Indeed, proliferative tuft cells occur in vivo both in fetal and in adult human intestine. Single mature proliferating tuft cells can form organoids that contain all intestinal epithelial cell types. Unlike stem and progenitor cells, human tuft cells survive irradiation damage and retain the ability to generate all other epithelial cell types. Accordingly, organoids engineered to lack tuft cells fail to recover from radiation-induced damage. Thus, tuft cells represent a damage-induced reserve intestinal stem cell pool in humans.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In Antibodies on 24 April 2024 by Rubio-Pérez, L., Frago, S., et al.
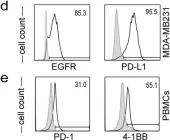
Immune checkpoint blockade has changed the treatment paradigm for advanced solid tumors, but the overall response rates are still limited. The combination of checkpoint blockade with anti-4-1BB antibodies to stimulate tumor-infiltrating T cells has shown anti-tumor activity in human trials. However, the further clinical development of these antibodies has been hampered by significant off-tumor toxicities. Here, we generated an anti-4-1BB/EGFR/PD-L1 trispecific antibody consisting of a triple-targeting tandem trimerbody (TT) fused to an engineered silent Fc region. This antibody (IgTT-4E1-S) was designed to combine the blockade of the PD-L1/PD-1 axis with conditional 4-1BB costimulation specifically confined to the tumor microenvironment (TME). The antibody demonstrated simultaneous binding to purified EGFR, PD-L1, and 4-1BB in solution, effective blockade of the PD-L1/PD1 interaction, and potent 4-1BB-mediated costimulation, but only in the presence of EGFR-expressing cells. These results demonstrate the feasibility of IgTT-4E1-S specifically blocking the PD-L1/PD-1 axis and inducing EGFR-conditional 4-1BB agonist activity.
-
FC/FACS
Tuft cells act as regenerative stem cells in the human intestine
Preprint on BioRxiv : the Preprint Server for Biology on 17 March 2024 by Huang, L., Bernink, J. H., et al.
ABSTRACT In mice, intestinal tuft cells have been described as a long-lived, post-mitotic cell type of which two distinct subsets have been identified, named tuft-1 and tuft-2 1 . By combining analysis of primary human intestinal resection material and intestinal organoids, we identify four distinct human tuft cell states, two of which overlap with their murine counterparts. We show that tuft cell development depends on the presence of Wnt ligands, and that tuft cell numbers rapidly increase upon interleukin (IL)-4 and IL-13 exposure, as reported previously in mouse 2–4 . This occurs through proliferation of pre-existing tuft cells, rather than through increased de novo generation from stem cells. Indeed, proliferative tuft cells occur in vivo both in fetal and in adult human intestine. Single mature proliferating tuft cells can form organoids that contain all intestinal epithelial cell types. Unlike stem- and progenitor cells, human tuft cells survive irradiation damage and retain the ability to generate all other epithelial cell types. Accordingly, organoids engineered to lack tuft cells fail to recover from radiation-induced damage. Thus, tuft cells represent a damage-induced reserve intestinal stem cell pool in humans.
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In Antibodies (Basel) on 24 April 2024 by Rubio-Pérez, L., Frago, S., et al.
Fig.2.D

-
FC/FACS
-
Collected and cropped from Antibodies (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 1