Susceptibility to life-threatening influenza increases with age, partly due to declining immunity. Frequency, phenotype and T-cell receptor (TCR) composition of influenza-specific CD8+ T-cells directed at the prominent A2/M158 influenza epitope change across the human lifespan.
We investigated longevity and mechanisms underlying age-related changes in influenza-specific TCR repertoires by performing longitudinal analyses in young and older adults across 7-12 years within A2/M158+CD8+ T-cells using peptide-HLA tetramers directly ex vivo. Paired TCRαβ-chains were used to track clonotypes over time within individuals.
Expanded public and private TCR clonotypes were long-lived but gradually declined over time. Loss of public clonotypes was initially compensated by expansions of clonotypes expressing public-associated features. Once these public-associated TCR clonotypes were abated in older adults, the void was filled by expansions of less similar private TCR clonotypes. Expanded older private TCR clonotypes also declined over time and were gradually replaced by other private TCR clonotypes with low similarity to public TCR clonotypes detected in adults.
Despite our relatively small cohort, we provided conclusive evidence that CD8+ T-cells to a single HLA-A2-restricted influenza-epitope are long-lived. However, dynamic changes occur at the clonotypic level, which eventually result in loss of public clonotypes, indicating that T-cell-based influenza vaccines are likely more effective in adults than older adults.
This research was supported by the National Health and Medical Research Council (#1173871, #1159272), the Australian Research Council (#190102704), European Union's Horizon 2020 (#792532), the University of Melbourne. Funders had no role in design, analysis or reporting of the study.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Product Citations: 22
In EBioMedicine on 1 May 2025 by van de Sandt, C. E., McQuilten, H. A., et al.
-
Immunology and Microbiology
In Haematologica on 1 May 2024 by Zeidler, A., Borbaran-Bravo, N., et al.
Mutations in the ELANE gene, encoding the neutrophil elastase (NE) protein, are responsible for most cyclic neutropenia (CyN) cases and approximately 25% of congenital neutropenia (CN) cases. In CN and in CyN, a median of 2.8% of CD34+ cells were early CD49f+ hematopoietic stem cells (eHSC) that did not express ELANE and thus escape from the unfolded protein response (UPR) caused by mutated NE. In CyN, the CD49f+ cells respond to granulocyte colony-stimulating factor (G-CSF) with a significant upregulation of the hematopoietic stem cell-specific transcription factors, C/EBPα, MLL1, HOXA9, MEIS1, and HLF during the ascending arm of the cycle, resulting in the differentiation of myeloid cells to mature neutrophils at the cycle peak. However, NE protein released by neutrophils at the cycle's peak caused a negative feedback loop on granulopoiesis through the proteolytic digestion of G-CSF. In contrast, in CN patients, CD49f+ cells failed to express mRNA levels of HSC-specific transcription factors mentioned above. Rescue of C/EBPα expression in CN restored granulopoiesis.
-
FC/FACS
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cardiovascular biology
CD8+ T-cell responses towards conserved influenza B virus epitopes across anatomical sites and age.
In Nature Communications on 29 April 2024 by Menon, T., Illing, P. T., et al.
Influenza B viruses (IBVs) cause substantive morbidity and mortality, and yet immunity towards IBVs remains understudied. CD8+ T-cells provide broadly cross-reactive immunity and alleviate disease severity by recognizing conserved epitopes. Despite the IBV burden, only 18 IBV-specific T-cell epitopes restricted by 5 HLAs have been identified currently. A broader array of conserved IBV T-cell epitopes is needed to develop effective cross-reactive T-cell based IBV vaccines. Here we identify 9 highly conserved IBV CD8+ T-cell epitopes restricted to HLA-B*07:02, HLA-B*08:01 and HLA-B*35:01. Memory IBV-specific tetramer+CD8+ T-cells are present within blood and tissues. Frequencies of IBV-specific CD8+ T-cells decline with age, but maintain a central memory phenotype. HLA-B*07:02 and HLA-B*08:01-restricted NP30-38 epitope-specific T-cells have distinct T-cell receptor repertoires. We provide structural basis for the IBV HLA-B*07:02-restricted NS1196-206 (11-mer) and HLA-B*07:02-restricted NP30-38 epitope presentation. Our study increases the number of IBV CD8+ T-cell epitopes, and defines IBV-specific CD8+ T-cells at cellular and molecular levels, across tissues and age.
© 2024. The Author(s).
-
Immunology and Microbiology
In Nature Immunology on 1 November 2023 by Van de Sandt, C., Nguyen, T. H. O., et al.
CD8+ T cells provide robust antiviral immunity, but how epitope-specific T cells evolve across the human lifespan is unclear. Here we defined CD8+ T cell immunity directed at the prominent influenza epitope HLA-A*02:01-M158-66 (A2/M158) across four age groups at phenotypic, transcriptomic, clonal and functional levels. We identify a linear differentiation trajectory from newborns to children then adults, followed by divergence and a clonal reset in older adults. Gene profiles in older adults closely resemble those of newborns and children, despite being clonally distinct. Only child-derived and adult-derived A2/M158+CD8+ T cells had the potential to differentiate into highly cytotoxic epitope-specific CD8+ T cells, which was linked to highly functional public T cell receptor (TCR)αβ signatures. Suboptimal TCRαβ signatures in older adults led to less proliferation, polyfunctionality, avidity and recognition of peptide mutants, although displayed no signs of exhaustion. These data suggest that priming T cells at different stages of life might greatly affect CD8+ T cell responses toward viral infections.
© 2023. The Author(s).
-
Homo sapiens (Human)
-
Immunology and Microbiology
Characterizing infection of B cells with wild-type and vaccine strains of measles virus.
In IScience on 20 October 2023 by Melot, L., Bankamp, B., et al.
Acute infection with measles virus (MeV) causes transient immunosuppression often leading to secondary infections. MeV infection of B lymphocytes results in changes in the antibody repertoire and memory B cell populations for which the mechanism is unknown. In this study, we characterize the infection of primary B cells with wild-type and vaccine strains of MeV. Vaccine-infected B cells were characterized by a higher percentage of cells positive for viral protein, a higher level of viral transcription and reduced cell death compared to wild-type infected cells, regardless of B cell subtype. Vaccine-infected cells showed more production of TNF-α and IL-10 but less production of IL-8 compared to wild-type infected cells. IL-4 and IL-6 levels detected were increased during both vaccine and wild-type infection. Despite evidence of replication, measles-infected B cells did not produce detectable viral progeny. This study furthers our understanding of the outcomes of MeV infection of human B cells.
-
FC/FACS
-
Immunology and Microbiology
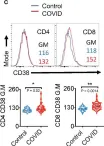
In Front Immunol on 6 October 2023 by Mahalingam, S. S., Jayaraman, S., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1