Detecting antigen-specific lymphocytes is crucial for immune monitoring in the setting of vaccination, infectious disease, cancer, and autoimmunity. However, their low frequency and dispersed distribution across lymphoid organs, peripheral tissues, and blood pose challenges for reliable detection. To address this issue, we developed a strategy exploiting the functions of tissue-resident memory T cells (T rm s) to concentrate target circulating immune cells in the skin and then sample these cells non-invasively using a microneedle (MN) skin patch. T rm s were first induced at a selected skin site through initial sensitization with a selected antigen. Subsequently, these T rm s were restimulated by intradermal inoculation of a small quantity of the same antigen to trigger the “alarm” and immune recruitment functions of these cells, leading to accumulation of antigen-specific T cells from the circulation over several days. In mouse models of vaccination, we show that application of MN patches coated with an optimized hydrogel layer for cell and fluid sampling to this skin site allowed effective isolation of thousands of live antigen-specific lymphocytes as well as innate immune cells. In a human subject with allergic contact dermatitis, stimulation of T rm s with allergen followed by MN patch application allowed the recovery of diverse lymphocyte populations that were absent from untreated skin sites. These results suggest that T rm restimulation coupled with microneedle patch sampling can be used to obtain a window into both local and systemic antigen-specific immune cell populations in a noninvasive manner that could be readily applied to a wide range of disease or vaccination settings.
Product Citations: 260
Preprint on MedRxiv : the Preprint Server for Health Sciences on 21 March 2025 by Jalili, S., Hosn, R. R., et al.
-
Immunology and Microbiology
Developing Monosodium Urate Monohydrate Crystals-Induced Gout Model in Rodents and Rabbits.
In Current Protocols on 1 March 2025 by Wang, Y., Zhang, Y., et al.
Gout is a chronic disease caused by the deposition of monosodium urate monohydrate (MSU) crystals within the body, particularly in one or more joints, which can lead to sudden severe attacks of pain, swelling, redness, and tenderness, known as gout flares. Historically termed the "disease of kings," gout is one of the oldest joint diseases and remains the most common form of inflammatory arthritis haunting humans in the 21st century. It is associated with cardiovascular, metabolic, and renal comorbidities and can lead to reduced mobility and impaired physical function and contributing to work absenteeism. Given its increasing global incidence, novel therapies for gouty arthritis disease are urgently needed. Experimental gout models are indispensable tools for deciphering disease pathogenesis and evaluating the efficacy and side effect of newly developed therapeutics at preclinical stage. Herein, we described a series of highly reproducible acute gout flare and air pouch models in rodents and rabbits that can be used to address various scientific questions relevant to pathological changes and immune responses during and after a gout attack. Animal gout flare models, elicited by MSU crystals, mimic the main histopathological features of human gouty arthritis, including damage to cartilage and joint swelling. Meanwhile, air pouch models serve as a tool to evaluate robust inflammatory cytokine secretion and neutrophil infiltration. This article provides a detailed description of procedures and troubleshooting tips required to reproducibly induce gout flare and air pouch models in animals and critically evaluate the pathogenesis of the disease. © 2025 Wiley Periodicals LLC. Basic Protocol 1: Preparation of monosodium urate crystalline Basic Protocol 2: Development of MSU-induced gout flare model in mice Support Protocol 1: Histological assessment of mouse ankle tissues Basic Protocol 3: Development of MSU-induced gout flare model in rats Basic Protocol 4: Development of MSU-induced gout flare model in rabbits Basic Protocol 5: Development and validation of reference articles in MSU-induced air pouch model in rats Basic Protocol 6: Development and validation of reference articles in MSU-induced air pouch model in mice Support Protocol 2: Flow cytometry of mouse neutrophils in air pouch lavage samples.
© 2025 Wiley Periodicals LLC.
Autocrine CXCL1-CXCR2 Signaling Mediates Leptomeningeal Resistance to Radiation Therapy
Preprint on BioRxiv : the Preprint Server for Biology on 27 February 2025 by Osman, A. M., Remšík, J., et al.
Leptomeningeal metastasis (LM) is a fatal neurological complication of cancer. Proton craniospinal irradiation (pCSI) has emerged as a promising life-prolonging intervention for LM patients, but the response to this treatment varies. Here, we aimed to characterize the molecular basis of pCSI resistance and response. Proteomic analysis of CSF collected from LM patients at baseline (before pCSI), and at multiple time points post-treatment, identified the CXC-motif chemokine, CXCL1, as associated with LM growth. Higher CXCL1 levels in the CSF prior to pCSI correlated with worse response to this treatment. To define the role of CXCL1 in LM, we established syngeneic mouse models of LM-CSI. We found that both metastatic cancer and host cells generate CXCL1. Genetic interruption of Cxcl1 expression in metastatic cancer, but not host cells, impaired cancer cell growth within the leptomeninges. Moreover, a subset of LM cancer cells expressed Cxcr2, the primary receptor for Cxcl1, and this population was enriched over time in the leptomeninges. Transcriptomic profiling of this rare population revealed an enrichment in pathways implicated in cell cycle progression. Finally, interruption of Cxcl1-Cxcr2 signaling with intrathecally-delivered Cxcr2 antagonist hampered LM growth and sensitized the cells to CSI. Our results demonstrate that the Cxcl1-Cxcr2 signaling axis mediates LM growth, and identifies a potential actionable intervention to improve response to pCSI and halt LM progression. One Sentence Summary CXCL1-CXCR2 axis is a potential actionable therapeutic target to halt leptomeningeal metastasis progression and enhance response to craniospinal irradiation.
-
Endocrinology and Physiology
The emerging fungal pathogenCandida aurisinduces IFNγ to colonize mammalian hair follicles
Preprint on BioRxiv : the Preprint Server for Biology on 18 January 2025 by Merrill, E. D., Prudent, V., et al.
Public health alarm concerning the emerging fungus Candida auris is fueled by its antifungal drug resistance and propensity to cause deadly outbreaks. Persistent skin colonization drives transmission and lethal sepsis although its basis remains mysterious. We compared the skin colonization dynamics of C. auris with its relative C. albicans , quantifying skin fungal persistence and distribution and immune composition and positioning. C. auris displayed a higher propensity to colonize hair follicles and avidly bound to human hair. While C. albicans triggered an effective sterilizing type 3/17 antifungal immune response driven by IL-17A/F-producing lymphocytes, C. auris triggered a type 1, IFNγ-driven immune response targeting hair follicles. Rather than promoting fungal clearance, IFNγ enhanced C. auris skin colonization by acting directly on keratinocytes impairing epithelial barrier integrity and repressing antifungal defense programs. C. auris exploits focal skin immune responses to create a niche for persistence in hair follicles.
Preprint on BioRxiv : the Preprint Server for Biology on 4 January 2025 by Elder, A. M., Fairchild, H. R., et al.
Summary Postpartum mammary gland remodeling after a pregnancy/lactation cycle is characterized by mechanisms of immunosuppression. Here we show that SEMA7A promotes PD-L1 expression in immune cells of the mammary tissue during involution. These same phenotypes are mimicked in the microenvironment of SEMA7A-expressing tumors, which partially respond to αPD-1/αPD-L1 treatments in vivo. However, cells that remain after treatment are enriched for SEMA7A expression. Therefore, we tested a novel monoclonal antibody that directly targets SEMA7A-expressing tumors, in part, by reducing SEMA7A-mediated upregulation of PD-L1. In vivo, the SEMA7A monoclonal antibody also reduces tumor growth or promotes complete regression of mouse mammary tumors, reduces the immunosuppressive phenotypes in the tumor microenvironment and restores cytotoxic T cells suggesting that SEMA7A may be a candidate for a novel immune-based therapy for breast cancer patients.
-
Mus musculus (House mouse)
-
Cancer Research
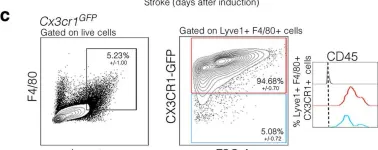
In Nat Commun on 30 November 2022 by Siret, C., van Lessen, M., et al.
Fig.7.C

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1