Chemical and biological stimulus screening in a hypogean actinomycete was used to elicit secondary metabolism. Optimal biosynthesis of bioactive natural products was identified using Multiplexed Activity Profiling for determining dose-dependent activity via six single-cell biological readouts. Bioactive extracts were fractioned to establish candidate compounds for isolation using Multiplexed Activity Metabolomics by correlating microtiter well-isolated phenotypes and extracted ion current peaks. This guided the isolation of four filipin polyene macrolides including a new metabolite filipin XV, an alkyl side-chain hydroxylated congener of the filipin chainin, with substantially attenuated cytotoxicity. Filipin-specific cytotoxicity was confirmed using flow cytometry and fluorescence microscopy.
© 2024. The Author(s).
Product Citations: 9
Multiplexed activity metabolomics for isolation of filipin macrolides from a hypogean actinomycete.
In The Journal of Antibiotics on 1 January 2025 by Froese, J. T., Balsamo, J. A., et al.
-
Homo sapiens (Human)
Signalling by senescent melanocytes hyperactivates hair growth.
In Nature on 1 June 2023 by Wang, X., Ramos, R., et al.
Niche signals maintain stem cells in a prolonged quiescence or transiently activate them for proper regeneration1. Altering balanced niche signalling can lead to regenerative disorders. Melanocytic skin nevi in human often display excessive hair growth, suggesting hair stem cell hyperactivity. Here, using genetic mouse models of nevi2,3, we show that dermal clusters of senescent melanocytes drive epithelial hair stem cells to exit quiescence and change their transcriptome and composition, potently enhancing hair renewal. Nevus melanocytes activate a distinct secretome, enriched for signalling factors. Osteopontin, the leading nevus signalling factor, is both necessary and sufficient to induce hair growth. Injection of osteopontin or its genetic overexpression is sufficient to induce robust hair growth in mice, whereas germline and conditional deletions of either osteopontin or CD44, its cognate receptor on epithelial hair cells, rescue enhanced hair growth induced by dermal nevus melanocytes. Osteopontin is overexpressed in human hairy nevi, and it stimulates new growth of human hair follicles. Although broad accumulation of senescent cells, such as upon ageing or genotoxic stress, is detrimental for the regenerative capacity of tissue4, we show that signalling by senescent cell clusters can potently enhance the activity of adjacent intact stem cells and stimulate tissue renewal. This finding identifies senescent cells and their secretome as an attractive therapeutic target in regenerative disorders.
© 2023. The Author(s).
-
FC/FACS
In Frontiers in Immunology on 18 April 2023 by Capellmann, S., Sonntag, R., et al.
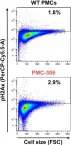
Mast cells (MCs) are immune cells of the myeloid lineage distributed in tissues throughout the body. Phenotypically, they are a heterogeneous group characterized by different protease repertoires stored in secretory granules and differential presence of receptors. To adequately address aspects of MC biology either primary MCs isolated from human or mouse tissue or different human MC lines, like HMC-1.1 and -1.2, or rodent MC lines like L138.8A or RBL-2H3 are frequently used. Nevertheless, cellular systems to study MC functions are very limited. We have generated a murine connective tissue-like MC line, termed PMC-306, derived from primary peritoneal MCs (PMCs), which spontaneously transformed. We analyzed PMC-306 cells regarding MC surface receptor expression, effector functions and respective signaling pathways, and found that the cells reacted very similar to primary wildtype (WT) PMCs. In this regard, stimulation with MAS-related G-protein-coupled receptor member B2 (MRGPRB2) ligands induced respective signaling and effector functions. Furthermore, PMC-306 cells revealed significantly accelerated cell cycle progression, which however was still dependent on interleukine 3 (IL-3) and stem cell factor (SCF). Phenotypically, PMC-306 cells adopted an immature connective tissue-like MCs appearance. The observation of cellular transformation was accompanied by the loss of Cdkn2a and Arf expression, which are both described as critical cell cycle regulators. The loss of Cdkn2a and Arf expression could be mimicked in primary bone marrow-derived mast cells (BMMCs) by sustained SCF supplementation strongly arguing for an involvement of KIT activation in the regulation of Cdkn2a/Arf expression. Hence, this new cell line might be a useful tool to study further aspects of PMC function and to address tumorigenic processes associated with MC leukemia.
Copyright © 2023 Capellmann, Sonntag, Schüler, Meurer, Gan, Kauffmann, Horn, Königs-Werner, Weiskirchen, Liedtke and Huber.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 24 January 2023 by Capellmann, S., Sonntag, R., et al.
Mast cells (MCs) are immune cells of the myeloid lineage distributed in tissues throughout the body. Phenotypically, they are a heterogeneous group characterized by different protease repertoires stored in secretory granules and differential presence of receptors. To adequately address aspects of MC biology either primary MCs isolated from human or mouse tissue or different human MC lines, like HMC-1.1 and -1.2, or rodent MC lines like L138.8A or RBL-2H3 are frequently used. Nevertheless, cellular systems to study MC functions are very limited. We have generated a murine connective tissue-like MC line, termed PMC-306, derived from primary peritoneal MCs (PMCs), which spontaneously transformed. We analyzed PMC-306 cells regarding MC surface receptor expression, effector functions and respective signaling pathways, and found that the cells reacted very similar to primary wildtype (WT) PMCs. In this regard, stimulation with MAS-related G-protein-coupled receptor member B2 (MRGPRB2) ligands induced respective signaling and effector functions. Furthermore, PMC-306 cells revealed significantly accelerated cell cycle progression, which however was still dependent on IL-3 and stem cell factor (SCF). Phenotypically, PMC-306 cells adopted an immature connective tissue-like MCs appearance. The reason for immortalization most likely is the loss of the two critical cell cycle regulators Cdkn2a /INK4A and Arf /p19, respectively. The loss of Cdkn2a and Arf expression could be mimicked in primary bone marrow-derived mast cells (BMMCs) by SCF supplementation strongly arguing for an involvement of KIT activation in the transformation process. Hence, this new cell line might be a useful tool to study further aspects of PMC function and to address tumorigenic processes associated with MC leukemia.
-
FC/FACS
-
Mus musculus (House mouse)
In The Journal of Biological Chemistry on 1 September 2022 by Balsamo, J. A., Penton, K. E., et al.
Natural products constitute and significantly impact many current anti-cancer medical interventions. A subset of natural products induces injury processes in malignant cells that recruit and activate host immune cells to produce an adaptive anti-cancer immune response, a process known as immunogenic cell death. However, a challenge in the field is to delineate forms of cell death and injury that best promote durable antitumor immunity. Addressing this with a single-cell chemical biology natural product discovery platform, like multiplex activity metabolomics, would be especially valuable in human leukemia, where cancer cells are heterogeneous and may react differently to the same compounds. Herein, a new ten-color, fluorescent cell barcoding-compatible module measuring six immunogenic cell injury signaling readouts are as follows: DNA damage response (γH2AX), apoptosis (cCAS3), necroptosis (p-MLKL), mitosis (p-Histone H3), autophagy (LC3), and the unfolded protein response (p-EIF2α). A proof-of-concept screen was performed to validate functional changes in single cells induced by secondary metabolites with known mechanisms within bacterial extracts. This assay was then applied in multiplexed activity metabolomics to reveal an unexpected mammalian cell injury profile induced by the natural product narbomycin. Finally, the functional consequences of injury pathways on immunogenicity were compared with three canonical assays for immunogenic hallmarks, ATP, HMGB1, and calreticulin, to correlate secondary metabolite-induced cell injury profiles with canonical markers of immunogenic cell death. In total, this work demonstrated a new phenotypic screen for discovery of natural products that modulate injury response pathways that can contribute to cancer immunogenicity.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cancer Research
In Front Immunol on 18 April 2023 by Capellmann, S., Sonntag, R., et al.
Fig.7.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1