Abstract Respiratory syncytial virus (RSV) is a significant cause of lower respiratory tract disease in young children and older adults. We designed a codon optimized mRNA vaccine, mRNA-1345, that encodes the RSV F-glycoprotein stabilized in the prefusion (preF) conformation and with a deletion at the cytoplasmic tail. mRNA-1345 protein expression was higher in vitro versus previous mRNA-based RSV vaccine candidates evaluated clinically. In rodent models, mRNA-1345 induced a robust neutralizing and preF-biased antibody response, a T helper 1-biased cellular response, and demonstrated dose-dependent protection against RSV challenge with no evidence of enhanced respiratory disease. These findings underscored the potential of mRNA-1345 as an effective RSV vaccine and are substantiated by clinical data demonstrating efficacy of mRNA-1345 in older adults.
Product Citations: 6
Preprint on Research Square on 12 December 2024 by Shaw, C. A., Stewart-Jones, G. B. E., et al.
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
T cell metabolic reprogramming in acute kidney injury and protection by glutamine blockade.
In JCI Insight on 22 June 2023 by Lee, K., Thompson, E. A., et al.
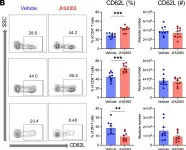
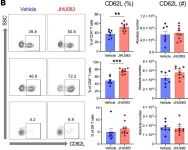
T cells play an important role in acute kidney injury (AKI). Metabolic programming of T cells regulates their function, is a rapidly emerging field, and is unknown in AKI. We induced ischemic AKI in C57BL/6J mice and collected kidneys and spleens at multiple time points. T cells were isolated and analyzed by an immune-metabolic assay. Unbiased machine learning analyses identified a distinct T cell subset with reduced voltage-dependent anion channel 1 and mTOR expression in post-AKI kidneys. Ischemic kidneys showed higher expression of trimethylation of histone H3 lysine 27 and glutaminase. Splenic T cells from post-AKI mice had higher expression of glucose transporter 1, hexokinase II, and carnitine palmitoyltransferase 1a. Human nonischemic and ischemic kidney tissue displayed similar findings to mouse kidneys. Given a convergent role for glutamine in T cell metabolic pathways and the availability of a relatively safe glutamine antagonist, JHU083, effects on AKI were evaluated. JHU083 attenuated renal injury and reduced T cell activation and proliferation in ischemic and nephrotoxic AKI, whereas T cell-deficient mice were not protected by glutamine blockade. In vitro hypoxia demonstrated upregulation of glycolysis-related enzymes. T cells undergo metabolic reprogramming during AKI, and reconstitution of metabolism by targeting T cell glutamine pathway could be a promising novel therapeutic approach.
-
FC/FACS
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 11 July 2022 by Ganesan, R., Bhasin, S. S., et al.
A major cause of cancer recurrence following chemotherapy is cancer dormancy escape. Taxane-based chemotherapy is standard of care in breast cancer treatment aimed at killing proliferating cancer cells. Here, we demonstrate that docetaxel injures stromal cells, which release protumor cytokines, IL-6 and G-CSF, that in turn invoke dormant cancer outgrowth both in vitro and in vivo . Single-cell transcriptomics shows a reprogramming of awakened cancer cells including several survival cues such as stemness, chemoresistance, as well as an altered tumor microenvironment with augmented pro-tumor immune signaling. IL-6 plays a role in cancer cell proliferation, whereas G-CSF mediates tumor immunosuppression. Pathways and differential expression analyses confirmed MEK as the key regulatory molecule in cancer cell outgrowth and survival. Antibody targeting of protumor cytokines (IL-6, G-CSF) or inhibition of cytokine signaling via MEK/ERK pathway using selumetinib prior to docetaxel treatment prevented cancer dormancy outgrowth suggesting a novel therapeutic strategy to prevent cancer recurrence.
-
Mus musculus (House mouse)
-
Cancer Research
In Frontiers in Immunology on 23 November 2021 by MacDonald, A., Lam, B., et al.
The phospholipid phosphatidylserine (PS) is naturally maintained on the cytoplasmic side of the plasma membrane. Independent of apoptosis, PS is redistributed to the surface of CD8 T cells in response to TCR-mediated activation. Annexin V (AnnV) is a protein known to bind PS with high affinity and has been effectively utilized to anchor antigen to the surface of CD8 T cells. To expand these studies, we aimed to exploit TCR activation driven PS exposure as a target to deliver cytokine, namely interleukin-2 (IL-2), to the surface of CD8 T cells. This was accomplished using a novel chimeric fusion protein of annexin V and interleukin 2 (AnnV-IL2). In vitro analysis revealed that AnnV-IL2 is able to specifically bind PS on the T cell surface following TCR stimulation. Consequently, AnnV-IL2 proved to be significantly more effective at enhancing T cell activation compared to recombinant IL-2. In vivo, AnnV-IL2 promotes robust expansion of antigen-specific cells capable of interferon gamma (IFNγ) production when administered following peptide vaccination. Importantly, upon antigen rechallenge, AnnV-IL2 treatment mice demonstrated a stronger secondary expansion, indicating durability of AnnV-IL2 mediated responses. Our data supports the use of AnnV-IL2 to modulate antigen-specific T cell immunity and demonstrates that the PS-AnnV axis is a feasible mechanism to target diverse cargo to CD8 T cells.
Copyright © 2021 MacDonald, Lam, Lin, Ferrall, Kung, Tsai, Wu and Hung.
-
Immunology and Microbiology
In Nature Communications on 16 July 2021 by Zhao, Z., Wang, H., et al.
Mucosal-associated Invariant T (MAIT) cells are recognized for their antibacterial functions. The protective capacity of MAIT cells has been demonstrated in murine models of local infection, including in the lungs. Here we show that during systemic infection of mice with Francisella tularensis live vaccine strain results in evident MAIT cell expansion in the liver, lungs, kidney and spleen and peripheral blood. The responding MAIT cells manifest a polarised Th1-like MAIT-1 phenotype, including transcription factor and cytokine profile, and confer a critical role in controlling bacterial load. Post resolution of the primary infection, the expanded MAIT cells form stable memory-like MAIT-1 cell populations, suggesting a basis for vaccination. Indeed, a systemic vaccination with synthetic antigen 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil in combination with CpG adjuvant similarly boosts MAIT cells, and results in enhanced protection against both systemic and local infections with different bacteria. Our study highlights the potential utility of targeting MAIT cells to combat a range of bacterial pathogens.
© 2021. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In JCI Insight on 22 June 2023 by Lee, K., Thompson, E. A., et al.
Fig.8.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 2
In JCI Insight on 22 June 2023 by Lee, K., Thompson, E. A., et al.
Fig.11.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 2